Abstract
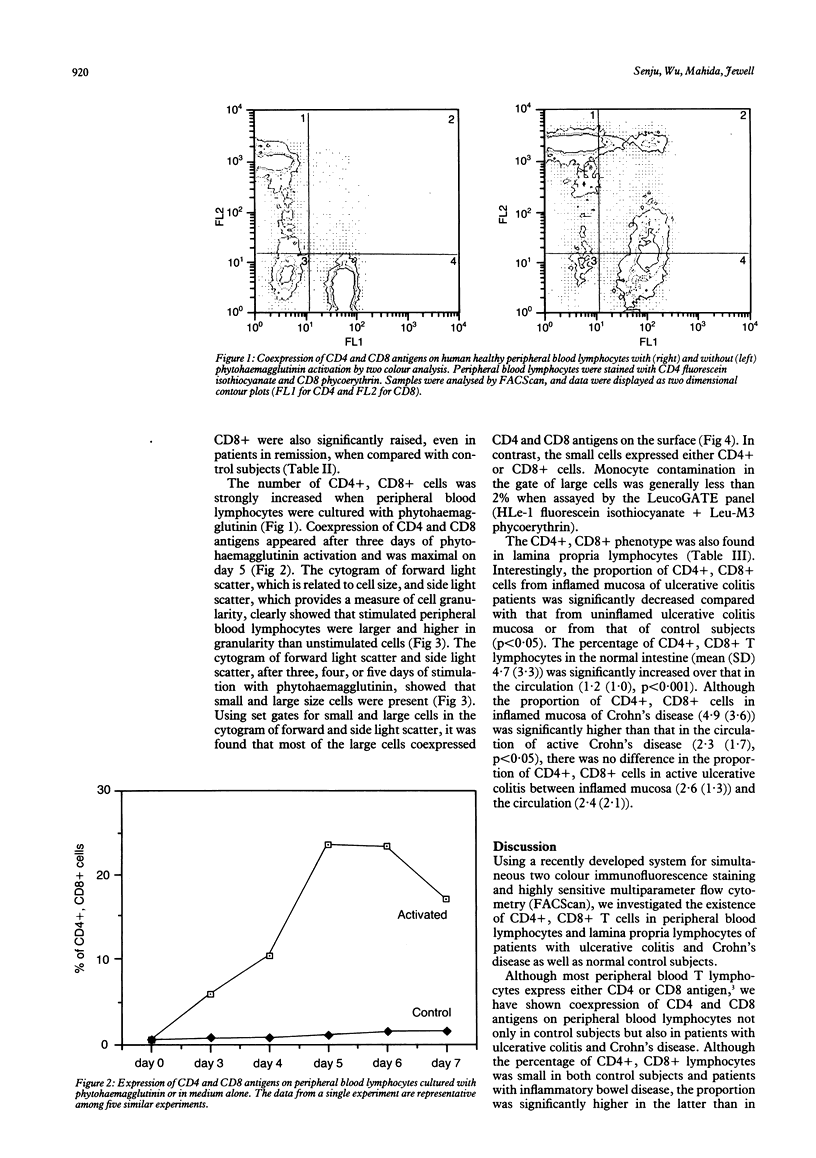
Using two colour immunofluorescence with fluorescein isothiocyanate and phycoerythrin labelled monoclonal antibodies and multiparameter flow cytometry, we investigated the coexpression of CD4 and CD8 antigens on peripheral blood lymphocytes and lamina propria lymphocytes of patients with ulcerative colitis and Crohn's disease and normal control subjects. Both the absolute number and the proportion of peripheral blood CD4+, CD8+ cells in inflammatory bowel disease were small but significantly increased compared with those in normal control subjects. Peripheral blood lymphocytes activated with phytohaemagglutinin showed appreciably increased coexpression of CD4+, CD8+. These CD4, CD8 positive cells were large and granular. Thus the increased number of peripheral blood CD4+, CD8+ cells in inflammatory bowel disease suggests that chronic immune activation occurs not only in the active state of the disease but also in remission. The proportion of CD4+, CD8+ cells in the lamina propria was greater than in peripheral blood in normal subjects, suggesting chronic immune stimulation of the local immune system. This was also seen in patients with Crohn's disease or inactive ulcerative colitis. The proportion of CD4+, CD8+ cells was, however, significantly less in the lamina propria of patients with active ulcerative colitis. Whether this implies a possible defect in mucosal immunoregulation in active ulcerative colitis cannot be determined from these results.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhan A. K., Reinherz E. L., Poppema S., McCluskey R. T., Schlossman S. F. Location of T cell and major histocompatibility complex antigens in the human thymus. J Exp Med. 1980 Oct 1;152(4):771–782. doi: 10.1084/jem.152.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Craig K. A., Schlossman S. F. Biosynthesis and surface expression of T8 by peripheral blood T4+ cells in vitro. J Immunol. 1986 Aug 15;137(4):1202–1207. [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Schlossman S. F. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985 Apr;134(4):2281–2286. [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Schlossman S. F. Discrete stages of human thymocyte activation and maturation in vitro: correlation between phenotype and function. Eur J Immunol. 1986 Jul;16(7):771–777. doi: 10.1002/eji.1830160710. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Hermanowicz A., Verhaar H. J., Ferguson D. J., Bernal A. L., Jewell D. P. Isolation of intestinal mononuclear cells: factors released which affect lymphocyte viability and function. Gut. 1985 Jan;26(1):60–68. doi: 10.1136/gut.26.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Allison J. P., Phillips J. H. Correlation of cell surface antigen expression on human thymocytes by multi-color flow cytometric analysis: implications for differentiation. J Immunol. 1986 Oct 15;137(8):2501–2507. [PubMed] [Google Scholar]
- Pallone F., Fais S., Squarcia O., Biancone L., Pozzilli P., Boirivant M. Activation of peripheral blood and intestinal lamina propria lymphocytes in Crohn's disease. In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987 Jun;28(6):745–753. doi: 10.1136/gut.28.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallone F., Montano S., Fais S., Boirivant M., Signore A., Pozzilli P. Studies of peripheral blood lymphocytes in Crohn's disease. Circulating activated T cells. Scand J Gastroenterol. 1983 Nov;18(8):1003–1008. doi: 10.3109/00365528309181833. [DOI] [PubMed] [Google Scholar]
- Raedler A., Fraenkel S., Klose G., Seyfarth K., Thiele H. G. Involvement of the immune system in the pathogenesis of Crohn's disease. Expression of the T9 antigen on peripheral immunocytes correlates with the severity of the disease. Gastroenterology. 1985 Apr;88(4):978–983. doi: 10.1016/s0016-5085(85)80017-2. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Richardson B., Kahn L., Lovett E. J., Hudson J. Effect of an inhibitor of DNA methylation on T cells. I. 5-Azacytidine induces T4 expression on T8+ T cells. J Immunol. 1986 Jul 1;137(1):35–39. [PubMed] [Google Scholar]
- Royer H. D., Acuto O., Fabbi M., Tizard R., Ramachandran K., Smart J. E., Reinherz E. L. Genes encoding the Ti beta subunit of the antigen/MHC receptor undergo rearrangement during intrathymic ontogeny prior to surface T3-Ti expression. Cell. 1984 Dec;39(2 Pt 1):261–266. doi: 10.1016/0092-8674(84)90003-5. [DOI] [PubMed] [Google Scholar]


