Abstract
Bradyrhizobium japonicum is a symbiotic bacterium that nodulates soybean. Critical for the infection and establishment of this symbiosis are the bacterial nodulation genes (nod, nol, noe), which are induced in the presence of plant produced isoflavones. Transcription of the nodulation genes is also controlled in a population density-dependent fashion. Expression of the nod genes is maximal at low population densities, and decreases significantly at higher culture densities. Population density control of the nodulation genes involves NolA and NodD2, both of which function in tandem to repress nod gene expression. An extracellular secreted factor (CDF) is known to mediate this repression. Here, we report that CDF is a novel signaling molecule, designated bradyoxetin, different from other Gram-negative quorum signals. The proposed structure of bradyoxetin is 2-{4-[[4-(3-aminooxetan-2-yl)phenyl](imino)methyl]phenyl}oxetan-3-ylamine. Interestingly, expression of bradyoxetin is iron-regulated, and is maximally produced under iron-starved conditions. Consistent with this, expression of the nodulation genes occurred in an iron-dependent fashion. Addition of iron to B. japonicum cultures at high optical densities resulted in decreased bradyoxetin production, and a concomitant reduction in nolA expression. A corresponding increase in nodY–lacZ expression was observed with iron treatment.
Bacteria are able to monitor changes in their environmental status and trigger appropriate responses to adjust and adapt to these variations. Responses to changes in population density are mediated by small molecule pheromones or autoinducers that function as determinants of population density (reviewed in refs. 1–3). These pheromones are present in low amounts at low population densities, accumulating as the bacterial population increases. Appropriate gene expression in response to these molecules is triggered when a threshold of molecules is present, or when the population is quorate. The most widespread form of signal molecules found among Gram-negative bacteria are the acyl homoserine lactones (AHLs) (reviewed in ref. 4). Generation and perception of these AHL signals involve members of the LuxR/LuxI protein families (5). In addition to AHLs, other signal molecules have been identified, and in many cases, function in concert with AHLs to elicit population density phenotypes. Non-AHL signals include Pseudomonas PQS, (2-heptyl-3-hydroxy-4-quinolone, ref. 6), Ralstonia solanacerium 3-OH PAME (3-hydroxypalmitic acid methyl ester, ref. 7), and Vibrio harveyii AI-2, a boron-containing furanone signal (8, 9).
Quorum control plays a large role in many bacterial-host interactions (10), including many bacterial plant infections (reviewed in ref. 3). In this regard, AHLs have been detected in many plant-associated bacteria, including members of the Rhizobiaceae (11–16). Rhizobia are able to form symbiotic associations with legume plants. To this end, mutants defective in AHL production and regulation are known to affect the ability of a Rhizobium to nodulate their respective host (16–19).
The nodulation genes (nod, nol, and noe) form a key component in the nodulation process. These genes encode for proteins that enzymatically produce a diffusible lipochitinoligosaccharide signal (i.e., Nod signal) molecule (reviewed in ref. 20). The Nod signal functions as a phytohormone and is able to elicit morphological changes when applied to the legume root. The nodulation genes are expressed in response to plant produced flavonoids. This induction is mediated, in part, by a LysR-type regulator NodD, which is common to all rhizobia (e.g., ref. 21). In the soybean symbiont, Bradyrhizobium japonicum, the response regulators NodW (22–24) and NwsB (25, 26), also positively activate the nodulation genes in response to the isoflavonoid genistein. Further control of the nodulation genes is provided by two additional proteins, a MerR-type protein NolA (27) and a LysR-type protein NodD2 (21). Together, NolA and NodD2 form a regulatory mechanism to repress the nodulation genes (28, 29). Both members are involved in the feedback regulation of the nodulation genes in response to levels of Nod signal (30). More recently, NolA and NodD2 were also shown to modulate nod gene expression in response to population density (31). Although induction of the nodulation genes is maximal in cultures of low population density, it is severely decreased in more dense cultures. NolA and NodD2 mediate this quorum phenotype, being maximally expressed at high population density, when the capacity to induce the nod genes is most reduced. This dependence on cell population is absent in a chromosomal nolA mutant (31). In a recent report, we demonstrated that quorum control of the nod genes also involves the response regulator NwsB, suggesting a key role for this protein in determining the ability of isoflavonoids to induce the nodulation genes (26).
Similar to other quorum systems, mediation of this population density dependence is achieved through the production of an extracellular signal, termed CDF (cell density factor), that accumulates with culture density (31). Preliminary analysis suggested that CDF was not an AHL. In our present study, chemical analysis of CDF supports a proposed structure containing oxetane rings; namely, 2-{4-[[4-(3-aminooxetan-2-yl)phenyl](imino)methyl]phenyl}oxetan-3-ylamine, which we have named bradyoxetin. Interestingly, expression of bradyoxetin is iron-regulated.
Experimental Methods
Bacterial Strains, Plasmids, and Culture Conditions.
The B. japonicum strains used in the study were B. japonicum USDA110 (Beltsville, MD), Bj110-41 (28) containing a nolA–lacZ fusion on plasmid pBGAlac4 and Bj110–1248 (i.e., nodD2–lacZ; ref. 21). For routine growth, strains were cultured on RDY medium (32). For isolation of CDF, strains were grown in minimal medium (33). Where necessary, chloramphenicol (50 μg/ml) and tetracycline (50 μg/ml) were applied.
Production and Isolation of CDF.
For analysis of CDF production, B. japonicum was cultured in minimal medium (33) at 30°C to various optical densities. Under the conditions used, an OD600 unit corresponds to ≈109 cells per ml. Bacterial cells were pelleted by centrifugation (8,000 × g) and the supernatant extracted with ethyl acetate (1:1 vol/vol). The ethyl acetate layer was concentrated by means of rotary evaporation. The sample was extracted with methanol, and the methanol phase applied to a C18 Sep-pak column (Phenomenex, Belmont, CA). Samples were eluted step wise from 0–100% methanol in 10% increments. Samples containing nolA inducing activity (see CDF Bioassay) were applied to a HPLC C18 column (Phenomenex) and eluted by using a 0–100% methanol gradient. Eluted fractions containing nolA activity were pooled and subjected to further purification using a 40–50% methanol gradient. The active fractions were dried and stored at −20°C. For structural analysis of CDF, cultures were grown to an OD600 = 2.0 and extracted as described above. The peak fraction containing nolA activity was concentrated and analyzed (see below).
To determine the effect of iron on CDF production, minimal medium was depleted in Fe3+ [i.e., Fe3+ depleted minimal medium (FDM medium)] by passage over a Chelex resin column (Bio-Rad). Cultures were inoculated into FDM medium or FDM medium supplemented with various concentrations of FeCl3. CDF production was determined by means of HPLC analysis as described above, or by using the CDF bioassay described below. All cultures were grown in disposable plastic labware that was pretreated with 10% HCl for more than 24 h before use.
CDF Bioassay.
Previous work demonstrated that CDF was able to induce a nolA–lacZ and nodD2–lacZ fusion (31). This formed the basis of our bioassay to detect CDF production. Briefly, Bj110-41 (i.e., nolA–lacZ) or Bj110-1248 (nodD2–lacZ) was cultured in minimal medium as previously described. The culture was diluted into fresh minimal medium, and allowed to grow for 5 h at 30°C in the presence of the test samples. β-Galactosidase activity was then measured as described (34).
Chemical Analysis of CDF.
The purified CDF was analyzed by both 1- and 2D NMR methods. The sample was dissolved in 1 ml of a 1:1 solution deuterated methanol (CD3OD)/water (D2O) and dried. This procedure was repeated once. The CDF was dissolved in about 0.7 ml of this same solution and analyzed. All NMR analyses were performed by using a Varian 500 MHz instrument. Both 1H and 13C 1D spectra were acquired. A series of 2D NMR experiments were needed to deduce the structure of CDF. These included gradient proton–proton COSY (gCOSY), total correlated spectroscopy (TOCSY), rotating frame Overhauser effect spectroscopy (ROESY), proton–carbon heteronuclear single quantum coherence spectroscopy (HSQC), and heteronuclear proton–carbon multiple bond coherence spectroscopy (HMBC). These spectra were acquired and processed by using the Varian software packages.
The mass spectrum of CDF was acquired by combined liquid chromatography (LC) mass spectrometry (MS) using a quadrupole time-of-flight (Q-TOF) hybrid mass spectrometer (Q-TOFII; Micromass, Manchester, U.K.) equipped with an electrospray source (Z-spray). The sample was dissolved in 20% methanol/water and applied to a C18 reverse phase capillary column, followed by a linear increase in the methanol concentration to 80% at a flow rate of 5 μl/min. The eluant from this column was infused into the mass spectrometer. A 3-kV potential (+ or −) was applied to the capillary, and nitrogen was used as both the drying and nebulization gas. Glu-Fibrinopeptide B was used as the calibration standard in the positive mode. For the MS analysis, the Q1 is operated in RF-only mode with all ions transmitted into the pusher region of the TOF analyzer, and the MS spectrum was recorded from m/z 400–2,000 with a 1-s integration time. The C/H/N ratio of CDF was determined by combustion analysis by the Chemical Analysis Laboratory at the University of Georgia.
An UV-visible spectrum of CDF was obtained. The sample was dissolved in 1 ml 1:1 methanol/water, and a spectrum was obtained by using a Shimadzu UV-2101PC UV-VIS spectrophotometer.
Detection of Siderophore Activity.
Siderophore activity was estimated by the liquid chrome azurol S (CAS) assay (35). Culture supernatants or CDF was mixed with CAS assay solution and incubated at room temperature. After 1 h, the absorbance at 630 nm was determined. Desferrioxamine mesylate (DFB) (Sigma) was used as a reference standard for siderophore activity.
Results
CDF Production Is Population Density Dependent.
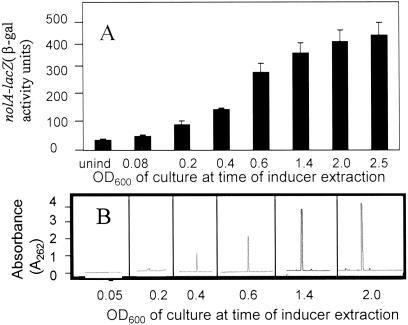
By using the protocol described in Experimental Methods, a single peak (termed CDF) capable of inducing nolA and nodD2 expression was isolated (data not shown). Consistent with the fact that NolA and NodD2 are involved in the repression of the nodulation genes, the addition of CDF to B. japonicum cultures reduced the ability of genistein to induce a nodY–lacZ fusion (compare 20-fold reduction for CDF treatment versus untreated). The CDF eluted from the C18 column at ≈40% methanol. To analyze CDF production as a function of population density, B. japonicum cultures were grown to various optical densities and extracted with ethyl acetate. These extracts were analyzed for their ability to induce a nolA–lacZ fusion in strain Bj110-41. As shown in Fig. 1A, samples derived from cultures grown to a high population density before extraction were the best inducers of nolA expression. This result is similar to that previously reported (31). Consistent with this, HPLC analyses of these extracts revealed a population-density-dependent increase in CDF production in these cultures (Fig. 1B). The highest production of CDF was detected with cultures grown to high optical density (OD600 > 2.0), with very little CDF detected at low culture densities (OD600 < 0.2). No CDF was found in control samples of uninoculated minimal medium.
Fig 1.
(A) nolA inducer (i.e., CDF) accumulates in a population-density-dependent fashion. B. japonicum cultures were grown to various optical densities, and ethyl acetate extracts were obtained from these cultures. These extracts were used to induce strain Bj110-41. (B) Analysis of CDF production. Extracts described above were subjected to HPLC analyses over a C18 column (Experimental Methods).
Structural Analysis of CDF.
CDF was analyzed by combined LC–MS. As described above for HPLC purification, the CDF eluted from the C18 reverse phase capillary column at 40% methanol as a single peak. The mass spectrum of this peak (not shown) had ions at [M+H]+ at m/z 324.0, [M+Na]+ at m/z 346.0, and [M-H2O+H]+ at m/z 306.0. These results indicate that the molecular weight of CDF is 323 mass units.
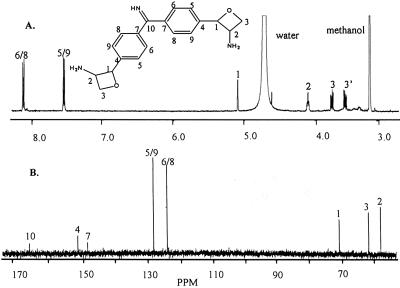
Combustion analysis of CDF gave a C/H/N ratio of C6.503 H6.880 N1.000. Multiplying this ratio by 3 and adding two oxygen atoms gives a likely empirical formula of C19H21N3O2 for CDF with a formula weight of 323. The UV spectrum of CDF (not shown) has a λmax of 271 nm, indicating an aromatic character. The proton spectrum, Fig. 2A, shows resonances at δ 8.13, 7.65, 5.18, 4.14, 3.82, and 3.61. Integration of this spectrum with normalization to the resonance at δ 5.18 gives a ratio of respective protons of 2:2:1:1:1:1. This ratio gives a minimal number of eight nonexchangeable protons. Given the above MS and combustion results, suggesting a total of 21 protons, the proton spectrum would indicate that CDF is a symmetrical compound containing 16 nonexchangeable and 5 exchangeable protons. The resonances at δ 8.18 and 7.65 suggest that CDF has aromatic character, a result that is consistent with the λmax of 271 nm. The fact that these two resonances appear as doublets supports the presence of p-substituted aromatic rings, and the molecular weight of 323 together with the above integration values supports the presence of two such rings with identical structures.
Fig 2.
The proton (A) and carbon (B) NMR spectra of CDF. The proposed structure and the proton and carbon NMR assignments are as shown.
The carbon spectrum, Fig. 2B, shows eight carbon resonances at δ 166.4, 151.5, 148.5, 128.3, 124.0, 71.3, 62.2, and 58.5. The intensities of the resonances indicate a relative number of two carbons each for the δ 128.3 and 124.0 resonances, one each for δ 71.3, 62.2, and 58.5, and one or less at δ 166.4, 151.5, and 148.5 for a total of 9 or 10 carbons. This relative number of carbons, together with the molecular ion of 323 and the combustion data, are consistent with the above indication that CDF is a symmetrical compound with 19 carbons. As with the proton spectrum, the carbon resonances at δ 128.3 and 124.0 are consistent with the ortho- and meta-carbons of the p-substituted aromatic rings, with the resonances at δ 151.5 and 148.5 caused by the substituted para-carbons of these rings. The assigned structure (explained further below) of CDF is also shown in Fig. 2.
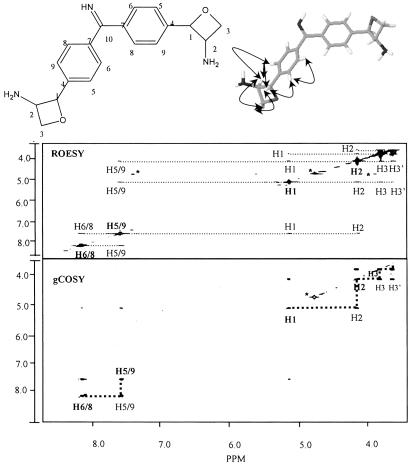
The gCOSY (Fig. 3), TOCSY (not shown), and HSQC (Fig. 4) revealed the various proton–proton correlations, coupling constants, and proton–carbon correlations and are summarized in Table 1. As mentioned above, the mass of CDF (m/z 323) supports the conclusion that CDF is a symmetrical molecule that contains two structurally equivalent p-substituted aromatic rings. These two rings account for 12 of the 19 carbons of CDF. The remaining carbons and their attached protons, except for the resonance at δ 166.4, exist as coupled spin system as shown by the gCOSY and TOCSY spectra. The gCOSY spectrum also has a low intensity signal between H1 (δ5.16) to H5/9 (δ7.65), which shows the presence of a weak long range coupling between these atoms. Thus, the single carbon atom at δ 166.4 is likely involved in bridging the two aromatic rings. Therefore, the remaining p-substituents would be comprised of six carbon atoms; three atoms for each of the two equivalent, and symmetrically located, p-substituent groups. In addition, the combustion and MS analysis show that there are three nitrogen atoms, two oxygen atoms, and five additional exchangeable protons. The HSQC spectrum (Fig. 4) shows that each p-substituent group has a proton at δ 4.14 that is attached to a carbon at δ 58.5, a result that is consistent with this carbon having an attached nitrogen. In addition, the HSQC spectrum shows that the p-substituent methylene protons are attached to a carbon at δ 62.2, which is consistent with this carbon having a attached oxygen. A structure consistent with these data would be an amino-substituted four-membered oxetane ring. If CDF is a symmetrical molecule as the data suggest, then one nitrogen, one oxygen, and two exchangeable protons would be part of each of these two equivalent p-substituents, and the remaining nitrogen and exchangeable proton in CDF would be part of the p group that bridges the two aromatic rings. This bridging group would then consist of one carbon, one nitrogen, and one proton; namely, an imine functional group. Thus, the data support a structure for CDF that consists of two aromatic rings bridged by an imino group, with each aromatic ring containing an identical p-substituted amino oxetane rings.
Fig 3.
The gCOSY and ROESY spectra of CDF together with the proposed structure showing the assignments and ROE contacts. In the gCOSY spectrum the bold dashed lines show the aromatic couplings from H5 to H6 and from H8 to H9, and of the proposed oxetane ring from H1 to H2 and from H2 to H3 and H3′. Also shown is the less intense weak long-range coupling from H1 of the oxetane ring to H5 and/or H9 of the aromatic ring. The ROESY spectrum shows the spacial interactions between the various protons. These interactions are depicted by the curved arrows in the structure shown at the top of the figure.
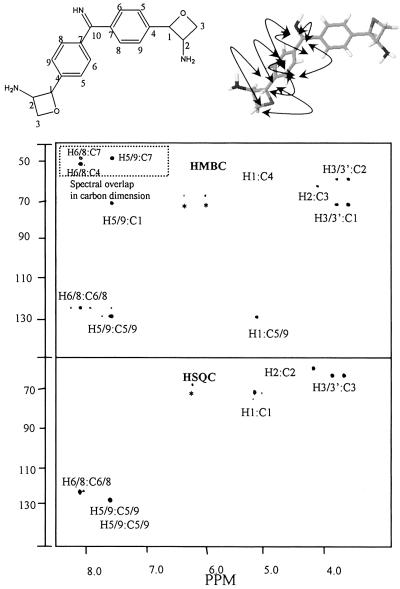
Fig 4.
The HSQC and HMBC spectra of CDF. The HSQC spectrum shows the interactions between the various protons and the carbons to which they are directly attached. Resonances for carbons 4, 7, and 10 do not appear because they have no attached protons. In the HMBC spectrum, the more intense spots are caused by the three-bond proton–carbon interactions. The less intense spots show several two-bond interactions. The dashed box is caused by the fact that, because of the spectral collection parameters, the resonances of the carbons, C4 and C7, in this region are folded into the spectrum. The chemical shifts for C4 and C7 are as indicated in Table 1.
Table 1.
Proton and carbon assignments, and coupling constants for CDF
| Position | Proton | Coupling constant | Carbon |
|---|---|---|---|
| 10 | – | – | 166.4 (1) |
| 4 | – | – | 151.5 (2) |
| 7 | – | – | 148.5 (2) |
| 5/9 | 7.65 (4) | J4,5 = 8.8 Hz | 128.2 (4) |
| 6/8 | 8.18 (4) | J4,5 = 8.8 Hz | 124.0 (4) |
| 1 | 5.16 (2) | J6,7 = 2.9 Hz | 71.3 (2) |
| 2 | 4.14 (2) | J6,7 = 2.9 Hz, J7,8 ≈ 6.6 Hz | 58.5 (2) |
| 3 | 3.82 (2) | J7,8 = 6.6 Hz, J8,8 = 11.5 Hz | 62.2 (2) |
| 3′ | 3.61 (2) | J7,8′ = 7.2 Hz, J8,8′ = 11.5 Hz |
The values in parentheses are the relative numbers of protons and carbons in CDF.
Rotation about the C7–C10 bonds is rapid on the NMR time scale, and because of the resulting time-averaged symmetry, C5 and C9 are chemically and magnetically equivalent, as are C6 and C8.
The structure of the two identical p-substituents was further supported by ROESY (Fig. 3), and HMBC (Fig. 4) NMR experiments. The ROESY spectrum shows that the proton at δ 5.16 had ROE contacts to those at δ 7.65, 4.14, and 3.82. The δ 7.65 contact of this δ 5.16 proton supports the conclusion that its corresponding carbon at δ 71.3 is attached directly to the aromatic ring. The HMBC spectrum (Fig. 4) confirms this conclusion in showing multiple two- and three-bond couplings of δ 71.3/7.65, δ 5.16/128.2, and δ 5.16/151.5. The fact that the δ 5.16 proton has a stronger ROE contact to the δ 3.82 methylene H3 proton and a weak contact to the δ 3.61 methylene H3′ proton (see ROESY spectrum), indicates that the rotation of this methylene carbon is limited; a result supporting the presence of an oxetane ring system. Thus, these data further support that CDF consists of two aromatic rings bridged by an imino group with each ring having a p substituent that is an amino oxetane ring. This proposed structure is shown in Fig. 3 and 4 together with the indicated ROE contacts and HMBC connectivities. The exact stereochemistry around C1 and C2 of this molecule is not known. A synthetic analog of an phenyl oxetane shows that a C1–C2 cis geometry has an H1/H2 coupling constant in the range of 8 Hz (36). However, this coupling for CDF is 2.95 Hz. This small H1/H2 coupling constant may mean that CDF has a trans C1/C2 configuration. Proof of the proposed structure for CDF will require a crystal structural determination. The International Union of Pure and Applied Chemistry (IUPAC) name for this structure, using the naming software from ACD Laboratories, is 2-{4-[[4-(3-aminooxetan-2-yl) phenyl](imino)methyl]phenyl}oxetan-3-ylamine. Oxetin, an antimetabolite from actinomycete fermentation, has been isolated and possesses the same oxetane ring system (37). Therefore, it is suggested that the common name for CDF be bradyoxetin. It should be noted that the structure shown for bradyoxetin, though consistent with the data, is a proposed structure.
CDF Production and nod Gene Activity Are Fe3+ Dependent.
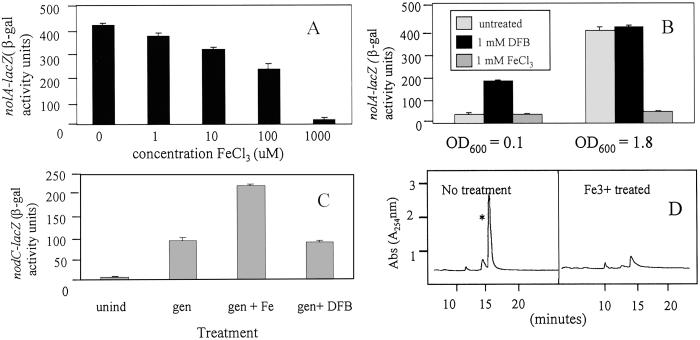
In addition to oxetin, the structure of bradyoxetin is also similar to an identified siderophore mugeneic acid (38). Given the role of Fe3+ in regulating siderophore production, we tested the ability of Fe3+ to affect both nod gene activity and CDF production. As an initial step to determine minimum Fe3+ requirements for growth in minimal medium, B. japonicum cultures were inoculated into Ferric depleted minimal medium (i.e., FDM) supplemented with various ferric iron concentrations (i.e., 0–200 μM Fe3+). No growth occurred in the presence of less than 10 μM Fe3+ (data not shown). All subsequent B. japonicum cultures were thus cultured in FDM medium supplemented with 25 μM Fe3+. We termed this Ferric minimum medium (i.e., FMM). To evaluate whether nod gene activity was Fe3+ dependent, bacteria cells were grown in FMM and subcultured in FMM supplemented with various concentrations of Fe3+. These cultures were then allowed to grow for an additional 24–36 h before being analyzed for nod gene activity. As shown in Fig. 5 A and B, the levels of nolA expression were dramatically reduced when the cells were treated with Fe3+. This repression occurred in a dose-dependent fashion and was observed in cultures grown to a high optical density (i.e., OD600 = 1.8). Maximal repression occurred in samples treated with 1 mM Fe3+. Repression of nolA expression was not seen in low population density cultures (i.e., OD600 = 0.1), likely because of the fact that the medium is iron sufficient under this condition (Fig. 5B). In contrast, cultures grown to high populations have likely exhausted the available iron, are iron-starved, and respond to the addition of exogenous iron. As a means to artificially lower the free iron levels, siderophore DFB was added to the cultures. As shown in Fig. 5B, the addition of DFB to B. japonicum cultures grown to low optical densities increased nolA–lacZ expression above that found in the untreated sample. Little effect of DFB on nolA–lacZ expression was noted when the siderophore was added to cultures of high population density. Modulation of nolA gene expression by iron was also assayed by using two B. japonicum iron uptake mutants (39, 40) that contain mutations to the iron-regulatory genes furR and irr respectively. nolA–lacZ expression was reduced in both these mutants (data not shown), supporting the notion that intracellular iron levels affect nolA expression. Consistent with the idea that nolA is involved in the repression of the nod genes, the addition of Fe3+ to high-population-density cultures of B. japonicum cells harboring a chromosomal nodC–lacZ fusion resulted in levels of nod gene expression that was consistently higher than that of cultures treated singly with genistein (Fig. 5C). Little or no effect of DFB was observed when added to these cultures. This result is in line with the fact that nolA is involved in the repression of nodC–lacZ expression, and that Fe3+ treatment results in lower NolA levels, and hence increased nodC–lacZ expression. To determine whether Fe3+-mediated nod gene activity is a result of effects on CDF production, B. japonicum cultures were grown to a high population density, and these cultures were either untreated or spiked with 200 μM Fe3+. In samples where Fe3+ was added, the levels of bradyoxetin production was significantly reduced when compared with samples where the cultures were not treated with Fe3+ (Fig. 5D). This result supports the notion that bradyoxetin production is Fe3+ dependent and that the effects on nod gene activity are a result of Fe3+-dependent reduction of bradyoxetin production. The structural similarity between bradyoxetin and mugeneic acid also led us to test the iron-binding properties of the molecule. CAS assays revealed very weak Fe3+ binding properties (1 mM of CDF provided 1 μM equivalent of DFB).
Fig 5.
Effect of iron on nolA expression. (A) B. japonicum cultures were grown to an OD600 = 1.8, and FeCl3 was added to these cultures to various concentrations. The cultures were grown 8–10 h, and nolA–lacZ expression was determined. (B) Comparison of iron and siderophore addition on nolA expression at high and low population densities. B. japonicum cultures were grown to either an OD600 = 0.1 or 1.8, and these cultures were treated with either 200 μM FeCl3 or 1 mM DFB. After 8–10 h incubation, nolA–lacZ activity was determined. Results represent means of three independent experiments. Standard deviation is reported. (C) Effect of iron and siderophore on nodC–lacZ expression. B. japonicum strain Bj110-573 harboring a nodC–lacZ fusion was induced with genistein in the presence or absence of either 200 μM FeCl3 or 1 mM DFB. Cultures were grown for 8–10 h, and nodC–lacZ expression was determined. Results represent means of three separate experiments ± SD. (D) Effect of iron on CDF production. B. japonicum cultures were grown to various optical densities and extracted with ethyl acetate (Experimental Methods). The extracts were analyzed by HPLC for CDF production.
Discussion
In this study, we demonstrate that B. japonicum produces a novel population density dependent inducer of the nolA gene that is not an AHL (31). We have given this compound the common name bradyoxetin. Several interesting features can be gleaned from the structure of bradyoxetin. For instance, a characteristic of the proposed structure is the four-membered oxetane ring. Related four-membered rings are found in a number of naturally occurring molecules, including the lactam ring of penicillin and the oxetane ring of taxol. The oxetane ring system present in bradyoxetin is identical to that of an antibiotic (i.e., Oxetin) found in actinomycete Streptomyces sp. OM2317 (37). Oxetin inhibited the growth of Bacillus subtilis and Pyricularia oryzae, and possessed herbicidal activity against alfalfa and turnip. An antibiotic function for bradyoxetin is intriguing given that NolA belongs to the family of MerR type regulators (27). Several MerR members are known to activate their target genes in response to toxic compounds, conferring protection to the bacterium against these stresses. In this connection, quorum molecules identified in Rhizobium are known to affect stationary phase survival, and to confer protection against salt, stresses, etc. (e.g., ref. 13). Therefore, a possible role for bradyoxetin in nodulation may be to provide an adaptive edge for B. japonicum, giving it a competitive advantage against other bacteria in the rhizosphere.
Interestingly, the expression of bradyoxetin is iron regulated. Expression of bradyoxetin is maximally expressed under iron-deficient conditions, and reduced under Fe3+ replete conditions. Both nolA and nodYABC expression were also affected under these conditions. The ability of iron to affect population density phenotypes has been observed with other quorum controlled systems. For example, in Pseudomonas, the oxidative stress response is regulated by both quorum-sensing and iron availability (41–43). Iron deficiency increases the expression of the lasI gene required for autoinducer production. In addition, excess or limiting iron can override the quorum control of the sodA gene (41). To this end, upstream of the quorum controlled sodA gene is, in addition to a quorum regulated Lux box, a putative “iron” box. The “iron” or “Fur” box is the site at which the iron associated Fur repressor binds (44). In our present study, an effect of iron on bradyoxetin signal production is also observed. However, unlike Pseudomonas aeruginosa sodA, no apparent B. japonicum iron box sequences (39, 40) were found upstream of either nolA or nwsB, a gene encoding a two-component regulator that mediates population density control of the nodulation genes (26). The absence of an iron box, however, does not preclude the fact that the gene may be regulated in an iron-dependent fashion. For instance, although Fur is known to bind and affect irr expression, no Fur box is identified upstream of irr (40).
The relationship between iron availability and quorum sensing is likely reflective of a common link between nutrient limitation and quorum sensing. Besides iron, carbon, and phosphate availability are also known to affect quorum sensing in Ralstonia solanacearum (45) and P. aeruginosa (46, 47). The fact that iron and bradyoxetin together affect nod gene expression may not be surprising. In previous work (31), we proposed that in the nodules, where the bacteria are contained within small compartments (i.e., symbiosomes), quorum control played a key role repressing the in planta expression of the nod genes. Iron forms a key component in the developing nodule and a high demand is placed on the plant to supply the necessary iron (48, 49). Most of the iron is sequestered and used in nitrogen assimilation enzymes including nitrogenase, nitrate reductase, and nitrite reductase. Therefore, the biological availability of iron in the nodule is at a premium, and the levels of “free” iron would be a viable means of affecting nod gene activity. The fact that the nod genes are repressed in nodules suggests that B. japonicum cells are likely faced with iron limited conditions in planta. Together, population and iron control of bradyoxetin in the nodule can result in increased signal production, and hence elevated NolA and NodD2 expression, and the subsequent repression of the nod genes. As determined by CAS assays, bradyoxetin does not appear to be a bona fide siderophore with high affinity Fe3+ binding capacity. However, it should be noted that the nodule provides a reducing environment and that ferrous iron is the likely source of iron in soybean nodules (50). These conditions may nullify the need for a high-affinity siderophore. Work by Wittenberg et al. (51) suggests the presence of a nodule-specific siderophore. It is possible that bradyoxetin may serve this function.
The role of iron has been described in many host–microbe interactions, including many animal pathogens (e.g., refs. 52–55). Elaborate regulatory mechanisms have been developed to acquire the necessary iron for maintenance of pathogenesis. It is interesting to note that many pathogens belong to the α-proteobacterium division. Of these pathogens, several (e.g., Brucella, Bartonella) exist in “symbiosome-like” intracellular vacuoles within the host cell. In this connection, a preliminary screen of extracts derived from different bacterial strains by using the nolA–lacZ reporter strain revealed nolA inducing activity in extracts of all Rhizobium strains tested (e.g., R. leguminosarum bv. viciae, Sinorhizobium meliloti, Rhizobium sp. NGR234, R. loti) but also other species in the α-proteobacterium division (data not shown). These results suggest that bradyoxetin-type molecules, while likely forming a new class of signaling molecules among the symbiotic bacteria, may also form a common signaling mechanism among the α-proteobacteria. Moreover, our results lend support to the idea that bradyoxetin-like molecules may form a critical signaling molecule in host-pathogen interactions.
Acknowledgments
We thank Steve Wilhelm for useful discussions, Mark O'Brian for providing the fur and irr mutants, and V. S. Kumar Kolli for the mass spectrometry analysis. This work was supported by National Science Foundation Grant MCB0108955 and Liphatech, Inc. (Milwaukee, WI), and, in part, by Department of Energy Grant DE-FG02-93ER20097 (to the Complex Carbohydrate Research Center).
Abbreviations
AHL, acyl homoserine lactone
CDF, cell density factor
LC, liquid chromatography
ROESY, rotating-frame Overhauser effect spectroscopy
TOCSY, total correlated spectroscopy
gCOSY, gradient proton–proton COSY
HSQC, proton–carbon heteronuclear single quantum coherence spectroscopy
HMBC, heteronuclear proton–carbon multiple bond coherence spectroscopy
CAS, chrome azurol S
DFB, desferrioxamine mesylate
FDM, Fe3+ depleted minimal medium
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Whitehead N. A., Barnard, A. M. L., Slater, H., Simpson, N. J. L. & Salmond, G. P. C. (2001) FEMS Microbiol. Rev. 25, 365-404. [DOI] [PubMed] [Google Scholar]
- 2.Kleerebezem M., Quadri, L. E. N., Kuipers, O. P. & de Vos, W. M. (1997) Mol. Microbiol. 24, 895-904. [DOI] [PubMed] [Google Scholar]
- 3.Loh J., Pierson, E., Pierson, L. S., III, Stacey, G. & Chatterjee, A. (2002) Curr. Opin. Plant Biol. 5, 285-290. [DOI] [PubMed] [Google Scholar]
- 4.Fuqua C. & Greenberg, E. P. (1998) Curr. Opin. Microbiol. 1, 183-189. [DOI] [PubMed] [Google Scholar]
- 5.Fuqua W. C., Winans, S. C. & Greenberg, E. P. (1994) J. Bacteriol. 176, 269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pesci E. C., Milbank, J. B. J., Pearson, J. P., Mcknight, S., Kende, A. S., Greenberg, E. P. & Iglewski, B. H. (1999) Proc. Natl. Acad. Sci. USA 96, 11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flavier A. B., Clough, S. J., Schell, M. A. & Denny, T. P. (1997) Mol. Microbiol. 26, 251-259. [DOI] [PubMed] [Google Scholar]
- 8.Surette M. G., Miller, M. B. & Bassler, B. L. (1999) Proc. Natl. Acad. Sci. USA 96, 1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Schauder, S., Potier, N., Van Dorsselaer, A., Pelczer, I., Bassler, B. L. & Hughson, F. M. (2002) Nature 415, 545-549. [DOI] [PubMed] [Google Scholar]
- 10.Hardman A. M., Stewart, G. S. A. B. & Williams, P. (1998) Antonie Leeuwenhoek 74, 199-210. [DOI] [PubMed] [Google Scholar]
- 11.Cha C., Gao, P., Chen, Y.-C., Shaw, P. D. & Farrand, S. K. (1998) Mol. Plant–Microbe Interact. 11, 1119-1129. [DOI] [PubMed] [Google Scholar]
- 12.Elasri M., Delorme, S., Lemangeau, P., Stewart, G., Laue, B., Glickmann, E., Oger, P. M. & Dessaux, Y. (2001) Appl. Environ. Microbiol. 67, 1198-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorne S. H. & Williams, H. D. (1999) J. Bacteriol. 181, 981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray K. M., Pearson, J. P., Downie, J. A., Boboye, B. E. A. & Greenberg, E. P. (1996) J. Bacteriol. 178, 372-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schripsema J., de Rudder, K. E. E., van Vliet, T. B., Lankhorst, P. P., de Vroom, E., Kijne, J. W. & van Brussel, A. A. N. (1996) J. Bacteriol. 178, 366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosemeyer V., Michiels, V. J., Verreth, C. & Vanderleyden, J. (1998) J. Bacteriol. 180, 815-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodelas B., Lithgow, J. K., Wisniewski-Dye, F., Hardman, A., Wilkinson, A., Economou, A., Williams, P. & Downie, J. A. (1999) J. Bacteriol. 181, 3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cubo M., Economou, A., Murphy, G., Johnston, A. W. B. & Downie, J. A. (1992) J. Bacteriol. 174, 4026-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniels R., de Vos, D. E., Desair, J., Raedschelders, G., Luyten, E., Rosemeyer, V., Verreth, C., Schoeters, E., Vanderleyden, J. & Michiels, J. (2002) J. Biol. Chem. 277, 462-468. [DOI] [PubMed] [Google Scholar]
- 20.Spaink H. P. (2000) Ann. Rev. Microbiol. 54, 257-288. [DOI] [PubMed] [Google Scholar]
- 21.Gottfert M., Holzhauser, D. & Hennecke, H. (1992) Mol. Plant–Microbe Interact. 5, 257-265. [DOI] [PubMed] [Google Scholar]
- 22.Gottfert M., Grob, P. & Hennecke, H. (1990) Proc. Natl. Acad. Sci. USA 87, 2680-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanjuan J., Grob, P., Gottfert, M., Hennecke, H. & Stacey, G. (1994) Mol. Plant–Microbe Interact. 7, 364-369. [Google Scholar]
- 24.Loh J., Garcia, M. & Stacey, G. (1997) J. Bacteriol. 179, 3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grob P., Michel, P., Hennecke, H. & Gottfert, M. (1993) Mol. Gen. Genet. 241, 531-541. [DOI] [PubMed] [Google Scholar]
- 26.Loh J., Lohar, D., Andersen, B. & Stacey, G. (2002) J. Bacteriol. 184, 1759-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadowsky M. J., Cregan, P. B., Gottfert, M., Sharma, A., Gerhold, D., Rodriguez-Quinones, F., Keyser, H. H., Hennecke, H. & Stacey, G. (1991) Proc. Natl. Acad. Sci. USA 88, 637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia M. L., Dunlap, J., Loh, J. & Stacey, G. (1996) Mol. Plant–Microbe Interact. 9, 625-635. [DOI] [PubMed] [Google Scholar]
- 29.Gillette W. K. & Elkan, G. H. (1996) J. Bacteriol. 178, 2757-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loh J. & Stacey, G. (2001) Mol. Microbiol. 41, 1357-1364. [DOI] [PubMed] [Google Scholar]
- 31.Loh J., Yuen-Tsai, J. P. Y, Welborn, A. & Stacey, G. (2001) Mol. Microbiol. 42, 37-46. [DOI] [PubMed] [Google Scholar]
- 32.So J.-S., Hodgson, A. L. M., Haughland, R., Leavitt, M., Banfalvi, Z., Nieuwkoop, A. J. & Stacey, G. (1987) Mol. Gen. Genet. 207, 15-23. [DOI] [PubMed] [Google Scholar]
- 33.Bergersen F. J. (1961) Aust. J. Biol. Sci. 14, 349-360. [Google Scholar]
- 34.Banfalvi Z., Nieuwkoop, A., Schell, M., Besl, L. & Stacey, G. (1988) Mol. Gen. Genet. 214, 420-424. [DOI] [PubMed] [Google Scholar]
- 35.Schwyn B. & Neilands, J. B. (1987) Anal. Biochem. 160, 47-56. [DOI] [PubMed] [Google Scholar]
- 36.Bach T., Schroder, J., Brandl, T., Hecht, J. & Harms, K. (1998) Tetrahedron 54, 4507-4520. [Google Scholar]
- 37.Omura S., Murata, M., Imamura, N., Iwai, Y., Tanaka, H., Furusaki, A. & Matsumoto, T. (1984) J. Antibiot. 37, 1324-1332. [DOI] [PubMed] [Google Scholar]
- 38.Drechsel H. & Jung, G. (1998) J. Peptide Sci. 4, 47-181. [DOI] [PubMed] [Google Scholar]
- 39.Hamza I., Hassett, R. & O'Brian, M. R. (1999) J. Bacteriol. 181, 5843-5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamza I., Qi, Z., King, N. D. & O'Brian, M. R. (2000) Microbiology 146, 669-676. [DOI] [PubMed] [Google Scholar]
- 41.Bollinger N., Hassett, D. J., Iglewski, B. H., Costerton, J. W. & McDermott, T. R. (2001) J. Bacteriol. 183, 1990-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassett D. J., Howell, M. L., Sokol, P. A., Vasil, M. L. & Dean, G. E. (1997) J. Bacteriol. 179, 14442-14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassett D. J., Ma, J.-F., Elkins, J. G., McDermott, T. R., Ochsner, U. A., West, S. E. H., Hunag, C.-T., Fredericks, J., Burnett, S., Stewart, P. S., et al. (1999) Mol. Microbiol. 34, 1082-1093. [DOI] [PubMed] [Google Scholar]
- 44.Escolar L., Perez-Martin, J. & de Lorenzo, V. (1999) J. Bacteriol. 181, 6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flavier A. B., Schell, M. A. & Denny, T. P. (1998) Mol. Microbiol. 28, 475-488. [DOI] [PubMed] [Google Scholar]
- 46.Hassett D. J., Charniga, L., Bean, K. A., Ohman, D. E. & Cohen, M. S. (1992) Infect. Immun. 60, 328-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brint J. M. & Ohman, D. E. (1995) J. Bacteriol. 177, 7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston A. W. B., Yeoman, J. K. H. & Wexler, M. (2001) Adv. Microb. Physiol. 45, 114-156. [DOI] [PubMed] [Google Scholar]
- 49.Ragland M. & Theil, E. C. (1993) Plant Mol. Biol. 21, 555-560. [DOI] [PubMed] [Google Scholar]
- 50.Moreau S., Day, D. A. & Puppo, A. (1998) Planta 207, 83-87. [Google Scholar]
- 51.Wittenberg J. H., Wittenberg, B. A., Day, D. A., Udvardi, M. K. & Appleby, C. D. (1996) Plant Soil 178, 161-169. [Google Scholar]
- 52.Ratledge C. & Dover, L. G. (2000) Ann. Rev. Microbiol. 54, 881-941. [DOI] [PubMed] [Google Scholar]
- 53.Almiron M., Martinez, M., Sanjuan, N. & Ugalde, R. A. (2001) Infect. Immun. 69, 6225-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mietzner T. A. & Morse, S. A. (1994) Annu. Rev. Nutr. 14, 471-493. [DOI] [PubMed] [Google Scholar]
- 55.James B. W., Mauchline, W. S., Fitzgeorge, R. B., Dennis, P. J. & Keevil, C. W. (1995) Infect. Immun. 63, 4224-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]