Abstract
Serotonin (5-HT), a major neurotransmitter, has a large number of G protein-coupled receptors in mammals. On activation by exposure to their ligand, 5-HT2 receptor subtypes increase IP3 levels and undergo desensitization and internalization. To visualize the receptor in cells during these processes, we have constructed a 5-HT2A-enhanced GFP (SR2-GFP) fusion receptor. We show that this fusion receptor undergoes internalization on exposure to its natural ligand, 5-HT. Because 5-HT2A receptors activate the phospholipase C pathway, we studied the effect of protein kinase C (PKC) on the internalization process and found that activation of PKC by its specific activator phorbol 12-myristate 13-acetate, in the absence of 5-HT, leads to internalization of the receptor. Moreover, inhibition of PKC by its inhibitor sphingosine in the presence of 5-HT prevents the internalization process, suggesting that activation of PKC is sufficient and necessary for the internalization of 5-HT2A receptors. We also show that SR2-GFP recycles back to the plasma membrane after 5-HT-dependent internalization, suggesting a mechanism for resensitization. In addition, receptors that have been internalized on addition of phorbol 12-myristate 13-acetate in the absence of 5-HT also recycle to the surface, with a time course similar to that seen after activation of the receptors by 5-HT. Our study suggests that 5-HT2A receptors internalize and return to the surface after both serotonin- and PKC-mediated processes. This study reveals a role for PKC in receptor internalization and also shows that 5-HT2A receptors are recycled.
Keywords: GFP, desensitization, resensitization
Ageneral feature of G protein-coupled receptors (GPCRs) is the existence of complex regulatory mechanisms that modulate receptor responsiveness. These mechanisms underlie important physiological phenomena such as signal transduction and plasticity. Many GPCRs also demonstrate rapid desensitization in the continued presence of agonists, due to uncoupling of the receptor from the G protein involved (1). In some systems, such as opiate and β2 adrenergic receptors, sequestration or internalization of receptors appears to occur as a part of the overall process of agonist-induced desensitization (2–9).
The phenomena of receptor desensitization and down-regulation are well described in 5-HT receptors (10–15). For example, phosphatidylinositol hydrolysis in bovine aorta mediated by 5-HT2 receptors is markedly attenuated after brief treatments with agonists (16). 5-HT2 receptor-induced Ca2+ mobilization is rapidly diminished after agonist treatment of rat C6BU-1 glioma cells, and rapid decreases in 5-HT-induced currents are also seen in Xenopus oocytes expressing 5-HT2A receptors (17, 18).
Sequestration or internalization of 5-HT2A receptors is also thought to occur as part of the overall process of agonist-induced desensitization of 5-HT2A receptors (19, 20). The process of resensitization would require the recovery of the ability of the receptor to couple to G proteins. Although it is not clear that internalization of desensitized receptors is necessary for recovery of their ability to couple to G proteins, it may be part of the resensitization process. We constructed a 5-HT2A-enhanced GFP (EGFP) fusion receptor that is functional and allows for easy visualization and possible purification of the receptor along the entire pathway involved in activation, desensitization, internalization, and resensitization. It is known that activation of 5-HT2A receptors leads to the hydrolysis of phosphatidyl inositol bisphosphate (PIP2) and produces IP3 and diacylglycerol, which in turn activates protein kinase C (PKC). It has also been shown by a number of indirect and direct studies that PKC is translocated, i.e., activated on stimulation of 5-HT2A receptors (21, 22). In many cases, the internalization phenomenon depends on the posttranslational modification of the receptor through phosphorylation of specific residues, suggesting potential roles for protein kinase A and PKC or some other kinase.
We investigated the role of PKC on the internalization of SR2-GFP in HEK293 cell line. We determined that after exposure to the ligand or activation of PKC, the receptor is internalized and then recycled back to the surface. These results suggest possible mechanisms for functional 5-HT2A receptors to become available again at the cell surface. The ligand-independent internalization and recycling of the receptors may also be of significance when other IP3-linked receptors are activated within the same cell. The easy visualization of this fusion receptor and purification using either the GFP tag or receptor-specific antibodies will allow us to study the processes involved in receptor activation, internalization, and recycling.
Materials and Methods
Materials.
The pSR2 plasmid, with the rat 5-HT2A receptor full-length cDNA construct, was obtained from David Julius (University of California, San Francisco). pEGFP-N1 vector was from CLONTECH. NcoI, BamHI, and EcoRI were from Amersham Pharmacia Biotech. DMEM, FCS, penicillin, streptomycin, lipofectamine, G418, OptiMEM, dNTPs, and Hanks' balanced salt solution were obtained from Life Technologies (Grand Island, NY). Vent polymerase and T4 DNA ligase were purchased from New England Biolabs. Rhod2-AM dye and pluronic were from Molecular Probes. Poly(DL)-Ornithine, serotonin (5-HT), cycloheximide, phorbol 12-myristate 13-acetate (PMA), d-sphingosine (synthetic), and calphostin C were from Sigma. Thirty-five-millimeter dishes and flasks were from Nalge Nunc. Coverslips (Gold seal cover glass, No. 1) were from Clay Adams.
Construction of SR2-GFP, the 5-HT2A-EGFP Fusion Receptor.
The 1.8-kb cDNA fragment containing the entire rat 5-HT2A receptor-coding region was obtained from the plasmid pSR2 by PCR. The stop codon of the 5-HT2A receptor-coding region was altered to obtain a BamHI site by PCR by using the appropriate primer and cloned in frame into pEGFP-N1 vector from CLONTECH by using EcoRI and BamHI restriction sites. The entire segment that was cloned into the vector was sequenced to ensure that mutations in the coding region were absent and that the receptor-GFP fusion was in frame.
Generation of HEK293 Cell Lines with Stable Expression of SR2-GFP.
HEK293 cells were maintained in DMEM supplemented with 10% FCS, penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37°C, 5% CO2. Cells were grown to 65–70% confluency in 35-mm dishes and transfected with 2 μg of SR2-GFP DNA by using 10 μg of lipofectamine in 1 ml of OptiMEM. After 2 days in culture, cells were selected with 1 mg/ml of the neomycin analogue G418, and 30 stable lines (SB series) expressing varying amounts of SR2-GFP receptors, as determined through fluorescence expression, were established. All further experiments were conducted with two of these lines, SB-1 and -2, which expressed high and medium levels of the fusion receptor, respectively.
Functional Studies on the SR2-GFP Fusion Receptor.
SB-1 or -2 cells were grown to 60–70% confluency on 35-mm dishes at 37°C and loaded with Rhod2-AM, a calcium-sensitive fluorescent dye in DMEM with pluronic (1:1) for 30 min at room temperature. Cells were then washed twice with Hanks' balanced salt solution (HBSS) with Ca2+/Mg2+ and incubated for 45 min at room temperature. To determine whether the 5-HT2A-EGFP receptors were active, cells were bathed in 1 ml of HBSS solution (with Ca2+/Mg2+) and exposed to different concentrations of 5-HT (200 nM, 1 μM, 10 μM, 100 μM). Fluorescence changes, before and after the addition of 5-HT, were monitored to determine the functional activation of the 5-HT2A-EGFP receptors. Nontransfected HEK293 cells served as control.
Internalization Studies.
Either SB-1 or -2 cells were used in all internalization studies.
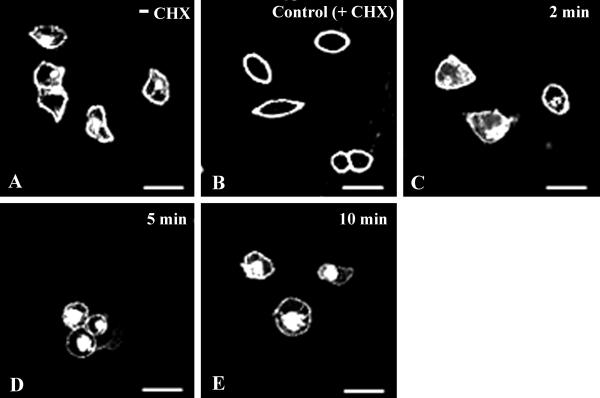
Cells were plated onto poly(DL)-ornithine- (20 μg/ml) coated coverslip dishes and grown to 60–70% confluency. After 24 h in culture, cells were washed twice with serum-free DMEM to remove endogenous 5-HT present in serum and incubated in DMEM supplemented with 10% dialyzed serum (5-HT free). Protein synthesis was then blocked with cycloheximide at 100 μg/ml for 5 h to eliminate fluorescence signals from any new receptors that could be synthesized and could be in traffic through the Golgi. At the end of 5 h, the fluorescence was observed only at the cell surface (Fig. 1 A and B).
Fig 1.
Kinetics of ligand-induced SR2-GFP internalization. (A) Representative image of SB-1 cells before cycloheximide treatment (see Materials and Methods). In the control (B), after cycloheximide treatment, most of the receptors were in the plasma membrane. Cells exposed to 10 μM 5-HT for 2 (C), 5 (D), and 10 min (E) are shown. Internalization of SR2-GFP began at 2 min (C) and was almost complete at 10 min (E). (Bar = 50 μm in Figs. 1–6.)
At the end of experimental procedures, the cells were fixed in 4% paraformaldehyde in PBS for 30 min, washed with PBS, and imaged by using a confocal microscope as detailed later. Typically, 100–150 cells were randomly chosen and imaged in any one experiment, and all experiments were repeated at least three times. In all experiments, cycloheximide was present until the cells were fixed.
To determine the time course of internalization, initial treatments of SB-1 or -2 cells were done as described above to clear their internal fluorescence. Cells were then incubated in media containing 10 μM 5-HT for 2, 5, or 10 min and then fixed and imaged. To determine the 5-HT dose–response of the fusion receptor, various concentrations of 5-HT (10 nM, 100 nM, 1 μM, 10 μM) were applied to SB-1 or -2 cells for 10 min, and the cells were then fixed and imaged as indicated before.
The effect of PKC on internalization of the 5-HT2A-EGFP receptor was determined at different concentrations of PMA (10, 50, or 100 nm), dissolved in ethanol. PMA, a specific activator of PKC, was applied to the cells (SB-1 or -2) for 10 min before fixation and imaging. To study the effect of inhibiting PKC on 5-HT2A-EGFP receptor internalization, the cells were preincubated for 10 min in 10 and 50 μM sphingosine (an inhibitor of PKC), after which 10 μM 5-HT was applied for a further 10 min (23). Cells were fixed and imaged as before.
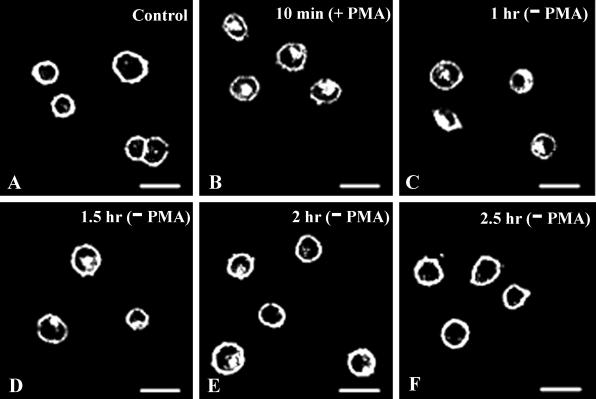
For recycling experiments, a 10-min pulse of 10 μM 5-HT or 100 nM PMA was applied. The cells were then washed and incubated at 37°C for different periods (10 min, 1 h, 1.5 h, 2 h, 2.5 h) in the absence of 5-HT or PMA before fixation and imaging.
Confocal Microscopy.
A laser-scanning confocal microscope (Model MRC1024, BioRad) attached to a Nikon inverted microscope (Nikon Eclipse TE300) and lasersharp software (Bio-Rad) were used for imaging. Laser power at 30% was used in all studies, and a 60× oil immersion objective (numerical aperture = 1.4) was used. GFP excitation/emission was achieved with a filter set (488/510 nm) designed for fluorescein detection. Images were processed (photoshop software, Adobe Systems, Mountain View, CA) by using identical values for contrast and brightness.
Results
Kinetics of SR2-GFP Internalization.
The 5-HT2A-EGFP (SR2-GFP) fusion receptor internalized on exposure to its ligand 5-HT. The 5-HT2A receptor is known to activate the IP3 second-messenger system on ligand binding with subsequent increase in intracellular Ca2+ levels. To determine whether the SR2-GFP is functional, increases in intracellular Ca2+ levels were monitored on application of 5-HT onto HEK293 cells expressing the receptor. On exposure to 5-HT, SB-1 and -2 cells showed an almost immediate increase in intracellular calcium, as seen using the Rhod2-AM dye (Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). Nontransfected HEK293 cells served as the control. On the other hand, application of 5-HT to Xenopus oocytes expressing the SR2-GFP receptor elicited the delayed onset oscillatory chloride currents characteristic of activation of the IP3/Ca2+ cascade in oocytes (data not shown). All these results indicate that, as expected, the 5-HT2A-EGFP fusion receptor is functional and couples to the IP3 second-messenger pathway.
To study the kinetics of SR2-GFP internalization, SB-1 or -2 cells were treated with cycloheximide for 5 h to block protein synthesis and thus clear the intracellular fluorescence of recently synthesized receptors. After such treatment, most of the fluorescence, i.e., receptors, were found on the plasma membrane (Fig. 1 A and B), and there was no detectable intracellular fluorescence. Subsequently, when cells were incubated with 10 μM 5-HT at 37°C for various times, the receptors were internalized and moved to intracellular compartments. The 5-HT-induced SR2-GFP internalization was quite rapid, and an increase in intracellular fluorescence was evident 2 min after exposure to 10 μM 5-HT (Fig. 1C). The majority of the receptors internalized within 10 min after exposure to 5-HT with an associated increase in intracellular fluorescence (Fig. 1E). Similar kinetics of internalization of the 5-HT2A receptors were reported by Berry et al. (19) on exposure of the wild-type receptor to its agonist quipazine. Thus, our results indicate that the 5-HT2A-EGFP construct internalizes on activation, and it does so with kinetics similar to those of the wild-type receptor.
Dose–Response Studies of SR2-GFP Internalization.
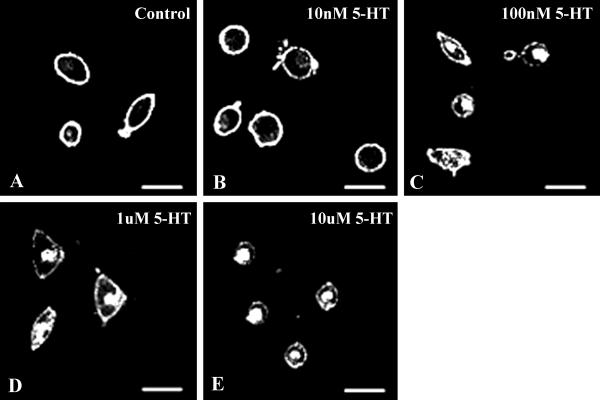
SB-1 or -2 cells treated with 100 μg/ml of cycloheximide for 5 h were incubated at 37°C in the presence of various concentrations of 5-HT (10 nM, 100 nM, 1 μM, 10 μM) for 10 min, at which time significant amounts of internalization were observed in the earlier experiment. After incubation in serotonin, the cells were fixed in 4% paraformaldehyde, and imaging was done with the confocal microscope. Internalization of the SR2-GFP began at 100 nM of 5-HT (Fig. 2C), and lower concentrations applied for 10 min or longer did not initiate any observable internalization (Fig. 2B).
Fig 2.
Dose–response of 5-HT-induced SR2-GFP internalization. (A) Most of the receptors at the plasma membrane after the initial cycloheximide treatment. Cells were incubated in various concentrations of 5-HT for 10 min and then imaged. At 10 nM 5-HT, the receptors were not sequestered (B). Internalization of SR2-GFP began at 100 nM of 5-HT (C) and increased with 5HT concentration, i.e., 1 μM (D) and 10 μM (E).
PKC Activation Is Sufficient and Necessary for Internalization of the SR2-GFP.
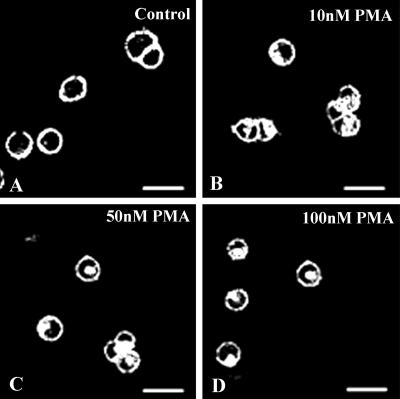
As already mentioned, binding of serotonin to 5-HT2A receptors activates the phospholipase C pathway and leads to the production of IP3 and diacylglycerol, which in turn activates PKC. We therefore examined whether PKC has a role in the internalization of 5-HT2A receptors by activating PKC directly, by using PMA. Addition of PMA to cycloheximide-treated SB-1 or -2 cells, in the absence of 5-HT, led to a significant amount of internalization of SR2-GFP. Internalization initiated on addition of 10 nM PMA (Fig. 3). This result shows that activation of PKC alone is sufficient for internalization of the receptor. When 10 or 50 μM of sphingosine (an inhibitor of PKC) was applied in the presence of 10 μM 5-HT, a marked decrease in the internalization of the receptor was observed (Fig. 4), suggesting that PKC activation is necessary for the internalization of the SR2-GFP. We have used another specific inhibitor of PKC, calphostin C (24). We have found that calphostin C (500 nM) also inhibits internalization of the receptor. Interestingly, we were unable to observe complete inhibition of receptor internalization by either sphingosine or calphostin C. The low levels of receptor internalization observed in the presence of sphingosine or calphostin C could be due to activation of other kinases, yet to be identified, or to another mechanism of internalization.
Fig 3.
Activation of PKC by PMA is sufficient for SR2-GFP internalization. (A) Control before application of PMA shows most of the receptors in the plasma membrane. Different concentrations of PMA were then applied to SB-1 cells for 10 min and imaged. Significant amounts of SR2-GFP internalization were observed from 10 nM (B) of PMA onwards and internalization increased with PMA concentration: 50 nM (C) and 100 nM (D).
Fig 4.
Internalization of the SR2-GFP is inhibited by sphingosine, an inhibitor of PKC. SB-1 cells were treated as described in the text. (A) Control; shows receptors localized to the cell surface. (B) Internalization of the receptor after applying 10 μM 5-HT for 10 min. Internalization of SR2-GFP by 10 μM 5-HT was inhibited by sphingosine: 10 μM (C) and 50 μM (D).
SR2-GFP Recycles Back to the Surface After 5-HT-Dependent Internalization.
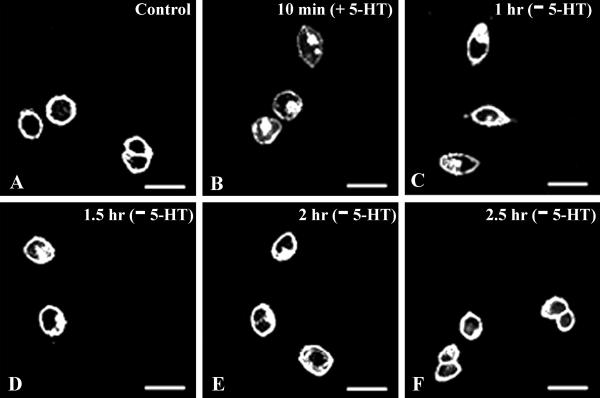
After ligand-dependent internalization, GPCRs exhibit different subcellular fates (25–29). Many GPCRs recycle back to the surface after internalization, and that process is believed to be important for resensitization of the receptor. To determine whether SR2-GFP recycles back to the surface after 5-HT-activated internalization, 10 μM 5-HT was applied for 10 min to SB-1 or -2 cells, after the initial cycloheximide treatment. The 5-HT was washed out, and the cells were then incubated for various times in the presence of cycloheximide and fixed. An increase in intracellular fluorescence and a decrease in surface fluorescence was observed after 5-HT exposure, i.e., the receptors were internalized (Fig. 5). When receptors were “chased” in the absence of 5-HT, they recycled back to the surface. An increase in the surface fluorescence and a decrease in the internal fluorescence of the cell were observed with time. After 2.5 h, most of the receptors were on the surface, and no significant amount of fluorescence was observed in the internal compartments of the cell. Because cycloheximide was present throughout the experimental procedure, new synthesis of the SR2-GFP was unlikely. This result suggests that SR2-GFP receptors that were internalized on activation by the ligand recycled back to the surface.
Fig 5.
SR2-GFP recycles back to the surface after ligand-mediated internalization. (A) Control before 5-HT application. (B) Internalization of the SR2-GFP receptor after 5-HT application (10 μM 5-HT for 10 min). Cells were washed free of 5-HT and incubated at 37°C for 1 h (C), 1.5 h (D), 2 h (E), and 2.5 h (F) and then imaged. Redistribution of the receptor was observed beginning at 1 h (C), and the receptor was found to recycle back to the surface almost completely after 2.5 h (F).
SR2-GFP Recycles to the Surface After 5-HT-Independent Internalization by Activation of PKC.
Because PMA alone caused SR2-GFP internalization, it was of interest to determine whether in this case the internalized receptor also recycled. Our results (Fig. 6) indicate that the PKC-activated SR2-GFP also returned to the surface and with a time course similar to that seen after exposure to 5-HT. These results suggest that activation and internalization of the SR2-GFP by PKC follows the same pathway that is activated by 5-HT.
Fig 6.
SR2-GFP recycles back to the surface after ligand-independent PKC-mediated internalization. (A) Control before application of PMA. SR2-GFP internalized in 10 min after exposure to 100 nM PMA (B). Cells were then washed free of PMA and incubated at 37°C for 1 h (C), 1.5 h (D), 2 h (E), and 2.5 h (F). The receptor recycled back to the surface after 2.5 h of incubation in the absence of PMA (F).
Discussion
Desensitization and resensitization of G protein-coupled receptors play important roles in many cellular functions. These processes depend on the availability of functional receptors at the cell surface, their mode of activation, and perhaps also on the cellular context. Agonist-induced internalization seems to be an important factor in the process of desensitization of many receptors, whereas resensitization results from recycling of internalized receptors, back to the cell surface or from the synthesis of new receptors.
In the present work, the C terminus of the full-length 5-HT2A receptor was tagged with a green fluorescent variant EGFP. Because the cytoplasmic tail of this receptor is around 86–98 amino acids, we assumed that the GFP moiety would not sterically hinder any of the processes involved during the internalization and recycling. As expected, this fusion receptor is inserted in the plasma membrane of HEK293 cells, and as reported for the native receptor, the GFP-tagged receptor undergoes internalization when serotonin (5-HT) is applied (19). We have also seen that the SR2-GFP colocalizes with the transferrin receptor during endocytosis, as reported earlier for the 5-HT2A receptor (19).
In HEK293 cells, the dose–response of the internalization process triggered by 5-HT was as expected for the 5-HT2A receptor subtype. Internalization began after 2-min activation, was complete within 10 min, and, after removal of 5-HT, the receptors recycled to the cell surface in 2.5 h. These results were observed in the absence of new receptor synthesis, which was blocked by cycloheximide. Therefore, our experiments provide strong visual evidence that the receptors can recycle back to the cell surface after being internalized in the normal course of activation, as has been reported for many other receptors (2, 27, 28). Moreover, the lack of discernible receptors within the cells, in the absence of agonist activation, suggests strongly that the nonactivated receptor is normally internalized and recycled only at very low levels, if at all. Receptors activated by PKC, in the absence of 5-HT, also recycle to the cell surface, and the kinetics of internalization and recycling are similar to those seen after activation by 5-HT. Thus, it appears that receptor internalization plays a role in the process of desensitization, whereas receptor recycling is a necessary step in the process of resensitization.
Because PKC is activated by diacylglycerol, which is generated on stimulation of 5-HT2A receptors, we studied the role of PKC on the internalization process. PKC can be activated directly by the phorbol ester PMA and this, even in the absence of 5-HT, is sufficient to cause internalization of 5-HT2A receptors. This result indicates that PKC plays a role in the internalization of receptors observed after application of their natural ligand 5-HT and suggests that, in the presence of 5-HT, blocking the activation of PKC would prevent receptor internalization. Indeed, we found that sphingosine (an inhibitor of PKC) inhibited greatly, although not completely, the internalization of SR2-GFP receptors. We therefore conclude that PKC activation is sufficient and necessary for the major pathway of internalization of this receptor. In a given cell, activation of PKC may occur via different types of receptors and coexpression of GPCRs in the same cell is not rare. This possibility implies that activation of the system by other IP3-linked receptors would cause the internalization of heterologous receptors within the same cell and raises a number of questions about the design of a system that would allow nonspecific internalization. It should be emphasized that our experiments are done in a cell line that normally does not express these receptors. So it is important to consider whether similar effects, e.g., crosstalk, are seen at all in vivo. Indeed, there are some examples of such crosstalk and subsequent integration of extracellular stimuli (30, 31). It is possible that the effects of activation of heterologous receptors might be confined to specific areas within a polarized cell, like the neurons. Nonetheless, internalization by activation of heterologous receptors within the same cell might play an important role in normal function. We believe that tagged receptors, like the one described here, could be very useful for studying these processes in vivo, if appropriately expressed.
There are a number of potential phosphorylation sites that could be the target for PKC-mediated phosphorylation in the third intracellular loop and the cytoplasmic tail of the 5-HT2A receptor. Phosphorylation of these residues may occur in the absence of 5-HT or by G protein activation, and that may be sufficient to internalize the receptor. The recycling of receptors internalized by 5-HT or by PMA suggests that the processes are similar. Because PMA is necessary and sufficient to cause internalization, this would suggest that internalization is also phosphorylation dependent. Recycling of the receptor might depend on dephosphorylation and could be an essential part of the resensitization process, although this has yet to be proven.
The results presented here suggest future directions toward identifying the residues involved in receptor internalization, residues that may be phosphorylated by 5-HT or by direct activation of PKC. All this will help us to understand the normal function and behavior of the receptor. The compartments to which the internalized receptors are directed under both ligand-dependent and independent activation are also of future interest.
Supplementary Material
Acknowledgments
We are grateful to Dr. David Julius (University of California, San Francisco) for the generous gift of the pSR2 plasmid with the rat 5-HT2A receptor full-length cDNA construct. We acknowledge Drs. Satyajit Mayor, Gaiti Hasan, K. S. Krishnan, and Jayant Udgaonkar for discussion and support. This work was supported by the National Centre for Biological Sciences, Tata Institute of Fundamental Research, India. R.M. was supported by the National Science Foundation.
Abbreviations
EGFP, enhanced GFP
PMA, phorbol 12-myristate 13-acetate
5-HT, serotonin
GPCR, G protein-coupled receptor
IP3, inositol trisphosphate
PKC, protein kinase C
PMA, phorbol 12-myristate 13-acetate
References
- 1.Sibley D. R., Benovic, J. L., Caron, M. G. & Lefkowitz, R. J. (1987) Cell 48, 913-922. [DOI] [PubMed] [Google Scholar]
- 2.Zastrow V. & Kobilka, B. K. (1992) J. Biol. Chem. 267, 3530-3538. [PubMed] [Google Scholar]
- 3.Trapaidze N., Keith, D. E., Cvejic, S., Evans, C. J. & Devi, L. A. (1996) J. Biol. Chem. 271, 29279-29285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sternini C., Spann, M., Anton, B., Keith, D. E., Bunnett, N. W., Zastrow, M. V., Evans, C. & Brecha, N. C. (1996) Proc. Natl. Acad. Sci. USA 93, 9241-9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueda H., Inoue, M. & Matsumoto, T. (2001) J. Neurosci. 21, 2967-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cvejic S. & Devi, L. A. (1997) J. Biol. Chem. 272, 26959-26964. [DOI] [PubMed] [Google Scholar]
- 7.Krueger K. M., Daaka, Y., Pitcher, J. A. & Lefkowitz, R. J. (1997) J. Biol. Chem. 272, 5-8. [DOI] [PubMed] [Google Scholar]
- 8.Yu S. S., Lefkowitz, R. J. & Hausdorff, W. P. (1993) J. Biol. Chem. 268, 337-341. [PubMed] [Google Scholar]
- 9.Menard L., Ferguson, S. S. G., Zhang, J., Lin, F. T., Lefkowitz, R. J., Caron, M. G. & Barak, L. S. (1997) Mol. Pharmacol. 51, 800-808. [PubMed] [Google Scholar]
- 10.Albert P. R. & Tiberi, M. (2001) Trends Endocrinol. Metab. 12, 453-460. [DOI] [PubMed] [Google Scholar]
- 11.Kinney G. G., Taber, M. T. & Gribkoff, V. K. (2000) Mol. Neurobiol. 21, 137-152. [DOI] [PubMed] [Google Scholar]
- 12.Massot O., Rousselle, J. C., Grimaldi, B., Cloez-Tayarani, I., Fillion, M. P., Plantefol, M., Bonnin, A., Prudhomme, N. & Fillion, G. (1998) Ann. N.Y. Acad. Sci. 861, 174-182. [DOI] [PubMed] [Google Scholar]
- 13.Gothert M., Propping, P., Bonisch, H., Bruss, M. & Nothen, M. M. (1998) Ann. N.Y. Acad. Sci. 861, 26-30. [DOI] [PubMed] [Google Scholar]
- 14.Albert P. R., Lembo, P., Storring, J. M., Charest, A. & Saucier, C. (1996) Neuropsychopharmacology 14, 19-25. [DOI] [PubMed] [Google Scholar]
- 15.Grant K. A. (1995) Drug Alcohol Depend. 38, 155-171. [DOI] [PubMed] [Google Scholar]
- 16.Pauwels P. J., Van Gompel, P. & Leysen, J. E. (1990) Life Sci. 47, 2009-2019. [DOI] [PubMed] [Google Scholar]
- 17.Kagaya A., Mikuni, M., Muraoka, S., Saitoh, K., Ogawa, T., Shinno, H., Yamawaki, S. & Takahashi, K. (1993) J. Neurochem. 61, 1050-1056. [DOI] [PubMed] [Google Scholar]
- 18.Woodward R. M., Panicker, M. M. & Miledi, R. (1992) Proc. Natl. Acad. Sci. USA 89, 4708-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry S. A., Shah, M. C., Khan, N. & Roth, B. L. (1996) Mol. Pharmacol. 50, 306-313. [PubMed] [Google Scholar]
- 20.Bhatnagar A., Willins, D. L., Gray, J. A., Woods, J., Benovic, J. L. & Roth, B. L. (2001) J. Biol. Chem. 276, 8269-8277. [DOI] [PubMed] [Google Scholar]
- 21.Kramer H. K., Poblete, J. C. & Azmitia, E. C. (1997) Neuropsychopharmacology 17, 117-129. [DOI] [PubMed] [Google Scholar]
- 22.Nash M. S., Wood, J. P. & Osborne, N. N. (1997) Exp. Eye Res. 64, 249-255. [DOI] [PubMed] [Google Scholar]
- 23.Soliakov L. & Wonnacott, S. (2001) Br. J. Pharmacol. 132, 785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi E., Nakano, H., Morimoto, M. & Tamaoki, T. (1989) Biochem. Biophys. Res. Commun. 159, 548-553. [DOI] [PubMed] [Google Scholar]
- 25.Marchese A. & Benovic, J. L. (2001) J. Biol. Chem. 276, 45509-45512. [DOI] [PubMed] [Google Scholar]
- 26.Volpicelli L. A., Lah, J. J. & Levey, A. I. (2001) J. Biol. Chem. 276, 47590-47598. [DOI] [PubMed] [Google Scholar]
- 27.Naik N., Giannini, E., Brouchon, L. & Boulay, F. (1997) J. Cell Sci. 110, 2381-2390. [DOI] [PubMed] [Google Scholar]
- 28.Tarasova N. I., Stauber, R. H., Choi, J. K., Hudson, E. A., Czerwinski, G., Miller, J. L., Pavlakis, G. N., Michejda, C. J. & Wank, S. A. (1997) J. Biol. Chem. 272, 14817-14824. [DOI] [PubMed] [Google Scholar]
- 29.Tsao P. I. & von Zastrow, M. (2000) J. Biol. Chem. 275, 11130-11140. [DOI] [PubMed] [Google Scholar]
- 30.Rocheville M., Lange, D. C., Kumar, U., Patel, S. C., Patel, R. C. & Patel, Y. C. (2000) Science 288, 154-157. [DOI] [PubMed] [Google Scholar]
- 31.Liang M., Eason, M. G., Jewell-Motz, E. A., Williams, M. A., Theiss, C. T., Dorn, G. W., 2nd & Liggett, S. B. (1998) Mol. Pharmacol. 54, 44-49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.