Abstract
Substance P (SP) excites large neurons of the nucleus basalis (NB) by inhibiting an inward rectifier K+ channel (Kir). The properties of the Kir in NB (KirNB) in comparison with the G protein-coupled Kir (GIRK) were investigated. Single-channel recordings with the cell-attached mode showed constitutively active KirNB channels, which were inhibited by SP. When the recording method was changed from the on-cell to the inside-out mode, the channel activity of KirNB remained intact with its constitutive activity unaltered. Application of Gβ1γ2 to inside-out patches induced activity of a second type of Kir (GIRK). Application of Gβ1γ2, however, did not change the KirNB activity. Sequestering Gβ1γ2 with Gαi2 abolished the GIRK activity, whereas the KirNB activity was not affected. The mean open time of KirNB channels (1.1 ms) was almost the same as that of GIRKs. The unitary conductance of KirNB was 23 pS (155 mM [K+]o), whereas that of the GIRK was larger (32–39 pS). The results indicate that KirNB is different from GIRKs and from any of the classical Kirs (IRKs). Whole-cell current recordings revealed that application of muscarine to NB neurons induced a GIRK current, and this GIRK current was also inhibited by SP. Thus, SP inhibits both KirNB and GIRKs. We conclude that the excitatory transmitter SP has two types of Kirs as its effectors: the constitutively active, Gβγ-independent KirNB channel and the Gβγ-dependent GIRK.
The nucleus basalis (NB) is located in the basal forebrain and contains a population of large cholinergic neurons that have axon projections to a wide area of brain regions including the cerebral cortex and the hippocampus (1). These cholinergic neurons play a crucial role in executing vital brain functions such as cognition and arousal (2), and degeneration of these neurons could be a major cause for the memory loss in Alzheimer's disease (3). Our group has been using these large neurons from the NB in culture together with neurons from the locus coeruleus (LC) as model neurons in the brainstem and the basal forebrain. One of the main objectives has been to elucidate the mechanism by which “slow transmitters” produce neuronal excitation through the modulation of the inward rectifier K+ channels (Kirs).
Substance P (SP) is a peptide transmitter (4) that induces slow excitation in peripheral and central neurons (5–8). Our initial studies revealed that the application of SP causes depolarization by inhibiting Kir current in NB neurons (will be referred to as the KirNB channel; refs. 9 and 10). Subsequent studies have shown that a considerable portion of this SP-induced inhibition of the KirNB is mediated through a transduction cascade consisting of Gαq/11, phospholipase Cβ1, and PKC (11, 12).
In parallel with these studies on KirNB channels, we have been investigating the characteristics of G protein-coupled Kirs (GIRKs) in noradrenergic neurons of the LC (13, 14). A large part of the GIRK mRNAs in LC neurons consists of GIRK1 and -2,§ and the channel properties of the GIRKs in LC are very similar to those of the cloned GIRK1/2 (15–17). One of the characteristics of the GIRKs in the LC is that the channel activity is regulated in a dual manner: the activity is enhanced by somatostatin and inhibited by SP (18, 19). Recently it has been reported that this type of dual regulation occurs in cloned GIRKs as well (20–22).
These data on the KirNB and the neuronal GIRK have raised a question. Does KirNB belong to the family of GIRKs? During the early stages of our investigations, we thought that the same Kir (later identified as GIRK) is involved in slow excitation and slow inhibition (13). More recently, however, we started to suspect that KirNB might be different from GIRK (12); the suspicion has arisen from the fact that KirNB channels are constitutively active, whereas GIRKs generally are not, and the single-channel conductance of KirNB is smaller than that of GIRKs (11). Nevertheless, this question has not been answered unambiguously.
Here we describe the results of analyzing the properties of KirNB channels in comparison to GIRKs. The analysis includes the sensitivity of the channels to G protein subunits. Our physiological data demonstrate that KirNB is unlikely to be a member of the GIRK family. Genetic identity of KirNB is yet to be determined. Nevertheless, both KirNB channels and GIRKs would play important roles in causing excitation in brain neurons, with each one of these two types of channels playing different roles under different circumstances. Some of the results were presented at a meeting.¶
Materials and Methods
Cultures of NB Neurons.
NB neurons were cultured by using reported procedures (23, 24). Briefly, 2- to 4-day-old postnatal Long–Evans rats (Charles River Breeding Laboratories) were anesthetized with ether, the forebrains were removed, and the rats were killed by decapitation. Brain slices (400-μm thickness) were made, and the NB was isolated under a dissecting microscope. The NB was incubated with papain (12 units/ml) before neurons were dissociated by trituration. Neurons were plated on a small well made inside a culture dish. The floor of the well was precoated with rat collagen and a glia feeder layer (24). Culture medium consisted of minimum essential medium with Earle's salt (88%, GIBCO/BRL) modified by adding 0.292 mg/ml l-glutamine/3.7 mg/ml NaHCO3/5 mg/ml d-glucose and was supplemented with heat-inactivated rat serum (2%, prepared in our laboratory)/10% heat-inactivated horse serum/10 μg/ml l-ascorbic acid/50 units/ml penicillin/50 μg/ml streptomycin/50 ng/ml 2.5 S nerve growth factor. The cultures were kept at 37°C and 10% CO2.
Electrophysiology.
Experiments were performed on NB neurons that were cultured for 10–16 days. We used only large neurons (diameter, ≈26 ± 3 μm; mean ± SD) likely to be cholinergic neurons (23).
For the cell-attached mode of patch clamp, the external bathing solution was 5-K Krebs, which contained 5 mM KCl, 146 mM NaCl, 2.4 mM CaCl2, 1.3 mM MgCl2, 5 mM Hepes-NaOH buffer, 11 mM d-glucose, and 0.0005 mM tetrodotoxin (pH 7.4). The patch-pipette solution was 155-KCl (or 155 K-gluconate) solution, containing 155 mM KCl (or K-gluconate), 2.4 mM CaCl2, 1.3 mM MgCl2, 5 mM Hepes-NaOH, and 0.0005 mM tetrodotoxin (pH 7.4). For the inside-out patch configuration, the bath solution (the cytoplasmic side solution) was 137 K-gluconate solution containing 137 mM K-gluconate, 10 mM NaCl, 2 mM MgCl2, 5 mM EGTA-KOH, 5 mM Hepes-KOH, 3 mM Na2-ATP, and 0.2 mM Na2-GTP (or GDP) (pH 7.2). The patch-pipette solution was the 155-KCl solution described above.
For whole-cell recordings, the external solution was 10-KCl Krebs, which contained 142 mM NaCl, 10 mM KCl, 2.4 mm CaCl2, 1.3 mM MgCl2, 5.0 mM Hepes-NaOH, 11 mM d-glucose, and 0.0005 mM tetrodotoxin (pH 7.4). The patch-pipette solution contained 141 mM K-gluconate, 10 mM NaCl, 5 mM Hepes-KOH, 0.5 mM EGTA, 0.1 mM CaCl2, 4 mM MgCl2, 3 mM ATP (Na2), and 0.2 mM GTPγS (pH 7.2).
The data were analyzed with PCLAMP programs. In single-channel recordings the overall frequency response was set at 2 kHz and digitized at 10 kHz. Membrane-potential values were corrected for the liquid-junction potential between the bath and the patch-pipette solutions. Drugs were applied by pressure ejection or by using a sewer pipe device.
Recombinant Gβ1γ2 and Gαi2 were a gift from Tohru Kozasa. The Gβ1γ2 was hexahistidine-tagged. The efficacy of the hexahistidine-tagged Gβ1γ2 in activating GIRKs was approximately the same as that of the Gβ1γ2 without the epitope (25), and therefore we will refer to the hexahistidine-tagged protein simply as Gβ1γ2. All experiments were performed at room temperature (20–24°C) except for the calyculin A experiment, which was done at a high bath temperature (≈30°C). Calyculin A was obtained from GIBCO/BRL or LC Laboratories (Woburn, MA). Statistical values are expressed as mean ± SEM unless otherwise stated.
Results
KirNB Channels Recorded with the Cell-Attached or Inside-Out Configurations.
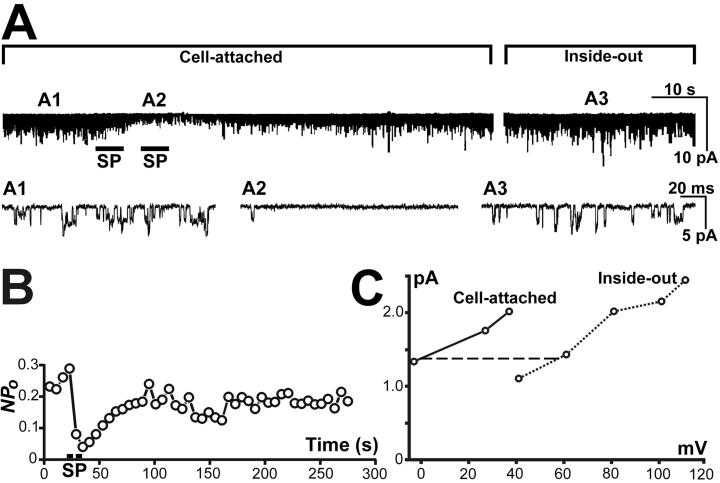
Fig. 1A illustrates the single KirNB channel activity recorded with the cell-attached mode from an NB neuron bathed in the 5-K Krebs solution. The holding potential was 27 mV more hyperpolarized than the resting potential. In almost all neurons, we observed channel activity that occurred spontaneously in the absence of any agonists. The chance of channel opening in the patch (Npo) of this spontaneous activity was 0.30 ± 0.04 (n = 26). Application of SP to the extra-patch region of the neuron caused a considerable reduction of the channel activity (Fig. 1 A and B), and the reduction recovered in 1 or 2 min at room temperature. Takano et al. (11, 12) obtained evidence indicating that the SP-induced inhibition of the single-channel activity of KirNB is the single-channel counterpart of the SP-induced inhibition of the Kir observed with the whole-cell configuration (9).
Fig 1.
Single-channel activity of KirNB from an NB neuron. (A) A1 and A2, Single-channel currents with cell-attached mode. The external solution was the 5-K Krebs solution. SP application (0.5 μM) induced a transient reduction of channel activity. The membrane potential of the patched region was 27 mV more hyperpolarized than resting potential. A3, Recorded with the inside-out configuration. During the gap between the two upper records (13 min), the 5-K Krebs bath solution was exchanged to the 137 K-gluconate solution (with GTP; total [K+], 155 mM), and the patch was excised. A holding potential of −91 mV was imposed (the liquid-junction potential difference between the agar bridge and the different bath solutions was taken into account). The downward direction represents openings of the channels. (B) SP application produced a transient decline of Npo; this graph was derived from the same patch as that in A. (C) Current–voltage relationships of KirNB channels (another patch different from A), recorded first in cell-attached mode (solid line), then followed by the inside-out mode (dotted line). The horizontal distance (dashed line) between the two curves should correspond to the resting potential.
After the recovery from the SP effect (Fig. 1A), the bath solution was exchanged from the 5-K Krebs to the 137 K-gluconate solution (the total [K+] is 155 mM), which would have depolarized the cell completely. The holding potential then was changed from −27 to −91 mV (the change of the liquid-junction potential between the bath solution and the ground agar bridge was taken into account), and the patch was excised (Fig. 1, A3, inside-out). Note that the channel activity with the inside-out mode at −91 mV (A3) looks very similar to that with the cell-attached mode (A1) at −27 mV. Fig. 1C shows an experiment in which the voltage–current relation was plotted during the cell-attached mode (solid line) and then during the inside-out configuration (dotted line). The horizontal distance (the dashed horizontal line) between the two I–V curves (at the level of zero potential in the on-cell curve) would correspond to the resting potential of the neuron. The resting potential determined in this way was −59.9 ± 4.2 mV (n = 8); the value is approximately what is expected for the neurons at [K]o = 5 mM (compare data at [K]o = 2.5 mM; ref. 23). Furthermore, the slopes of the two curves in Fig. 1C are quite similar. These results in Fig. 1 strongly suggest that the same channels are producing the constitutive activity both in the cell-attached inside-out configurations.
Recovery from the SP-Induced Suppression of KirNB Is Caused by Dephosphorylation.
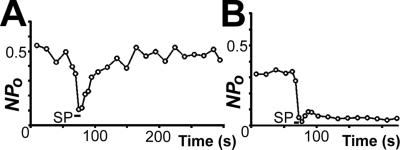
With the cell-attached configuration, the SP-induced suppression of the KirNB activity recovered spontaneously within 1 or 2 min (Fig. 1B). To observe the role of dephosphorylation in this recovery process, we applied calyculin A, an inhibitor of protein phosphatase 1 and 2A. In this experiment the bath temperature was raised to 29.4 ± 0.4°C to accelerate the recovery. In control neurons (treated with 0.1% DMSO for 20–90 min) the SP-induced suppression of the channel activity recovered to the 99.9 ± 10.7% (n = 7) level of the control in 1 min and to the 114 ± 7.8% (n = 3) level in 3 min (Fig. 2A). By contrast, neurons treated with calyculin A (0.1 μM in 0.1% DMSO for 20–100 min) recovered much more slowly; the recovery was only 8.8 ± 2.5% (n = 7) in 1 min and 37.2 ± 11% (n = 5) in 3 min (Fig. 2B). These data agree with that of our previous experiment using okadaic acid (inhibitor of mainly phosphatase 2A; ref. 11). The result is consistent with the idea that the activity of KirNB is suppressed by phosphorylation and recovered by dephosphorylation.
Fig 2.
Effects of calyculin A pretreatment on the SP-induced inhibition of the KirNB channel. The cell-attached single-channel recording was used. (A) Control neuron: SP effect on the Npo for KirNB. The NB neuron was pretreated with DMSO (0.1%) for ≈80 min. (B) SP effect on an NB neuron pretreated with 0.1 μM calyculin A (in 0.1% DMSO) for ≈45 min. The bath temperature was ≈29°C.
Single-Channel Property of KirNB with the Inside-Out Mode.
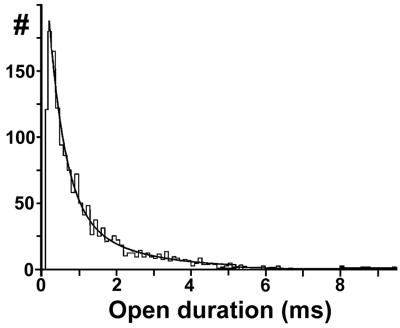
Because KirNB channels can be recorded with the inside-out configuration, single-channel properties of the KirNB channel were analyzed by using the inside-out mode. Open-time histograms were fitted well by 2 exponentials (Fig. 3). As shown in Table 1, the mean open time of the KirNB channel was 1.1 ms, which is similar to that of cloned GIRK1/2 (15–17). In contrast, the single-channel conductance of KirNB (23 pS) is certainly smaller than that for the GIRK in LC or that for the cloned GIRK1/2 (32–35 pS) (14, 17).
Fig 3.
Open-time histogram of KirNB channels obtained with the inside-out mode at −91 mV. The continuous curve represents the sum of two exponentials with af = 0.519, τf = 0.408 ms, and τs = 1.71 ms. The open time (To) = 1.03 ms.
Table 1.
Single-channel characteristics of KirNB
| af | τf | τs | To | γ |
|---|---|---|---|---|
| 0.44 ± 0.04 | 0.41 ± 0.09 ms | 1.7 ± 0.22 ms | 1.1 ± 0.16 ms | 23 ± 0.5 pS |
n = 8. The channel properties were measured by using the inside-out mode. [K+]o = 155 mM; holding potential = −91 mV; af, relative area of the fast exponential of the open-time histogram; τf, time constant of the fast exponential; τs, time constant of the slow exponential. Mean open time (To) is af × τf + ([1 − af] × τs). γ, single-channel chord conductance.
KirNB Is Not Activated by βγ.
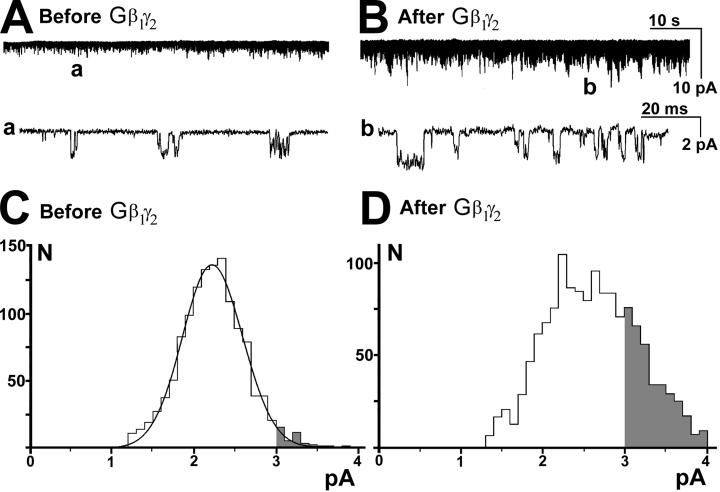
The characteristic feature of GIRKs is that the channel activity depends on the presence of Gβγ subunits (26, 27). We performed experiments to determine whether the KirNB activity depends on the presence of Gβγ. Fig. 4A shows the constitutively active KirNB channels recorded by using the inside-out configuration. We then applied recombinant Gβ1γ2, which caused a considerable increase in channel activity (Fig. 4B). The lower records (a and b) show that before the application of the protein (a), channels with ≈2-pA amplitude predominated, whereas after application of the protein, larger channels (≈3 pA) emerged (b). The amplitude histograms, before (Fig. 4C) and after (Fig. 4D) the Gβ1γ2 application, reveal that the difference caused by Gβ1γ2 is the emergence of channels that are larger than 3 pA in amplitude (shaded areas in C and D). It is known that the single-channel chord conductance of neuronal GIRKs in LC is 32 pS ([K]o = 156 mM, at −95 mV; ref. 14), which would produce a mean single-channel current of ≈2.9 pA under the present conditions. Thus, Fig. 4 C and D suggest an emergence of GIRK activity by Gβγ rather than an increase in KirNB activity.
Fig 4.
Both KirNB and GIRKs exist in NB neurons. Single-channel recordings with the inside-out configuration are shown. The holding potential = −91 mV. (A) KirNB channels showing constitutive activity. (B) Application of recombinant Gβ1γ2 protein (10 nM) to the inside-out patch activated additional channels with the amplitude larger than that of KirNB. The record was taken 5 min after the application of the protein. (C and D) Amplitude histograms of single-channel currents before and after the Gβ1γ2 application. C shows the constitutively active KirNB channel before the Gβ1γ2 application, and D shows a mixture of both KirNB and GIRKs after Gβ1γ2 application. Note the large increase in the number of channels with amplitude >3 pA, as illustrated by the shaded area of the histograms. The amplitude of short-duration channels cannot be determined accurately, because it takes a certain time for the current to reach a steady-state value. The current amplitude histograms thus were made after omitting the channels with the open times that were less than half of the mean open time (39). Record time was 33.1 s in C and 9.6 s in D.
To quantify the above assessment, we evaluated changes in channel frequency in two amplitude windows: 1.5–1.7 and 3.3–3.5 pA. Almost all channels within the 1.5- to 1.7-pA range can be regarded as representing KirNB, whereas almost all the channels within the 3.3- to 3.5-pA range would belong to GIRKs (Fig. 4 C and D). The frequency of channels that occupy the window between 1.5- and 1.7-pA amplitude did not change significantly by the introduction of Gβ1γ2 protein (Table 2), indicating that Gβ1γ2 did not alter the frequency of KirNB channels. On the other hand, a 20-fold increase in the frequency occurred in channels that fell within the 3.3- to 3.5-pA range (Table 2). The results indicate that the activity of to the KirNB channel did not change significantly by Gβ1γ2, whereas new channels of larger amplitude (GIRKs) emerged after the application of Gβ1γ2.
Table 2.
Effect of Gβ1γ2 on channel activity
| Amplitude, pA | Activity before Gβγ, openings per s | Activity after Gβγ, openings per s |
|---|---|---|
| 1.5–1.7 | 5.8 ± 0.88 | 5.6 ± 2.1 |
| 3.3–3.5 | 0.19 ± 0.05 | 3.8 ± 1.3 |
n = 8. Data were recorded with the inside-out mode at −91 mV. Short-duration openings were eliminated (see Fig. 4 legend). Almost all channels within 1.5–1.7 pA would represent KirNB, whereas almost all channels within 3.3–3.5 pA would belong to GIRKs (see Results). Frequency of the channel activity within 1.5–1.7 pA (KirNB) was not increased by the Gβ1γ2 (paired t test, P = 0.9). In contrast, the frequency of the channels in the 3.3- to 3.5-pA range (GIRK) was increased markedly (paired t test; P < 0.001).
KirNB Is Not Inhibited by Gαi2-GDP.
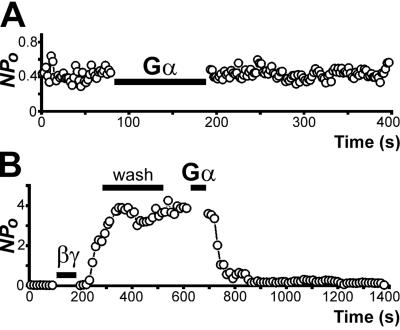
The activity of GIRK is initiated by the Gβγ subunits and terminated by sequestration of Gβγ with Gαi (GDP form; ref. 28). We therefore tested whether the KirNB activity is turned off by recombinant Gαi2-GDP. In Fig. 5A, constitutive activity of KirNB was occurring in an inside-out patch. Application of Gαi2-GDP, however, did not alter the channel activity (Npo). In some patches, there was an irregular drifting of Npo over a time scale of several minutes. On the average, however, Npo was not changed by Gαi: Npo before Gαi2 application was 0.63 ± 0.27 (n = 5), and Npo after Gαi2 application was 0.71 ± 0.27 (n = 5). As a positive control, we tested the potency of Gαi2 (Fig. 5B). We first activated the GIRK by applying Gβ1γ2. A subsequent application of Gαi2-GDP terminated the activity effectively (n = 5).
Fig 5.
(A) Application of Gαi2-GDP does not lower the KirNB activity. Gαi2-GDP (≈80 nM) was applied to an inside-out patch of an NB neuron. (B) Gβ1γ2 (≈40 nM) activated GIRKs, resulting in an increase in Npo. Subsequent washing did not alter the channel activity, but application of Gαi2-GDP (≈125 nM) effectively decreased the GIRK activity. The cytoplasmic side solution was the 137 K-gluconate solution (total [K+], 155 mM) with GDP. The holding potential was −91 mV. In the experiment of applying Gαi2-GDP such as in A, the concentration of the Gαi2 was ≈80–150 nM. In the experiment of the type in B, the concentration of Gβ1γ2 was ≈20–50 nM, and that of Gαi2-GDP was ≈50–125 nM.
How does the sequestration of Gβγ by Gα take place? In agreement with Logothetis et al. (28), once the GIRK activity was initiated by Gβγ, washing out of Gβγ did not lower the GIRK activity (Fig. 5B). With LC neurons, the GIRK activity, once initiated by Gβγ, lasted, after thorough washing of Gβγ, as long as we kept recording for up to ≈30 min (29). The result suggests that the Gβγ, once anchored to the membrane with the prenylation moiety of Gγ, cannot be removed easily by washing. The Gα subunit, also anchored to the membrane, is able to remove the βγ from the GIRKs. These considerations suggest that if the constitutive activity of KirNB were caused by the activity of the endogenous Gβγ located inside the membrane, the KirNB activity still would have been terminated by the Gαi2-GDP.
The result of the experiment in Fig. 5, therefore, demonstrates that KirNB, unlike GIRK, is not influenced by the presence or absence of Gβγ inside the membrane, indicating that the constitutive activity of KirNB is genuine and is not caused by activity of the endogenous Gβγ.
Single-Channel Properties of GIRK.
The above-described experiments indicate that in NB neurons there are two different Kirs: the GIRK, the activity of which depends on Gβγ, and the KirNB channel, which is constitutively active. Single-channel properties of the GIRK, however, cannot be determined easily because of the concomitant robust activity of the KirNB. We nevertheless measured the open time of the GIRKs within the 3.0- to 3.5-pA range; the channels within this window would be little-contaminated by KirNB (compare Fig. 4 C and D). The open time was 1.2 ± 0.14 ms (n = 8), a value similar to the open time for cloned GIRK1/2 (15–17).
In contrast, it is impossible to evaluate the single-channel conductance of the GIRK in NB. According to our single-cell RT-PCR study on cultured neurons, mRNAs for GIRK1 and GIRK2 were the most frequently observed members of the GIRK family both in NB and LC neurons.§ Therefore, the single-channel conductance of the GIRK in NB could be similar to that of the GIRK in LC or the cloned GIRK1/2 (32–35 pS; refs. 14 and 15–17).
SP Inhibits GIRKs as Well: Whole-Cell Recording.
Koyano et al. (18) observed that in LC neurons the activity of Kirs, which in retrospect would have corresponded to GIRKs, was inhibited by SP. Subsequently, Velimirovic et al. (19) observed that somatostatin activates GIRKs in LC neurons, whereas SP inhibits GIRKs that are being activated by somatostatin. The GIRK in LC neurons thus is dually modulated in the opposite direction.
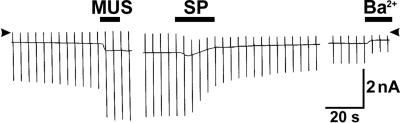
Is the GIRK in NB neurons also dually modulated in the opposite direction? To answer this question, GIRKs were fully activated by muscarine (30), presumably through the m2-muscarinic receptor (31). In the experiment in Fig. 6, we recorded the whole-cell currents in NB neurons. The pipette was loaded with 0.2 mM GTPγS. First, there was a spontaneous gradual increase in conductance, presumably caused by GTPγS. Application of muscarine produced an additional increase in Kirs (presumably GIRKs). A subsequent application of SP (0.3 μM) caused a fairly rapid irreversible inhibition of the GIRK current. The larger the GIRK current elicited (spontaneous plus muscarine-induced), the larger the current inhibition by SP, suggesting that the same channels that were activated (by GTPγS and muscarine) were inactivated by SP (data not shown). In conclusion, GIRKs in large neurons of the NB are dually regulated in the opposite direction; the inhibitory transmitter activates the GIRK, whereas SP inhibits the GIRKs that are being activated.
Fig 6.
SP-induced inhibition of GIRK in an NB neuron recorded with the whole-cell configuration. The internal solution contained 0.2 mM GTPγS. The holding potential was −79 mV. Arrowheads indicate the zero current level. Muscarine (MUS, 20 μM) activated GIRKs. A subsequent application of SP (0.3 μM) caused an inhibition of the GIRK. BaCl2 (100 μM) was applied at the end of the experiment to block the remaining K+ currents.
Discussion
KirNB Channel.
By using whole-cell recordings Stanfield et al. (9) found that SP inhibits a Kir current in NB neurons, and this inhibition causes a depolarization. Next, Takano et al. (11, 12), using the cell-attached single-channel mode, observed that a constitutively active inward rectifier channel, designated here as KirNB, is the single-channel counterpart of the SP-sensitive Kir. This SP-induced inhibition of KirNB is transduced through a cascade, starting with the Gq/11 activation, leading to the PKC-induced phosphorylation (11, 12). Despite the elucidation about its physiology, we were not certain about the identity of the KirNB. The KirNB channel was constitutively active, but mere constitutive activity does not exclude the possibility that KirNB belongs to the GIRK family, because the endogenous Gβγ could be present constantly, causing a spontaneous GIRK activity.
In this article we analyze the properties of KirNB channels using the inside-out configuration. The open time of KirNB was short (1.1 ms), and in this respect the KirNB channel is almost the same as the GIRK. However, the single-channel conductance of KirNB was 23 pS (at −91 mV, with [K]o = 155 mM); this is smaller than the single-channel conductance of GIRK in LC neurons or that of GIRK1/2 (32–35 pS; refs. 14 and 17).
Application of Gβγ to inside-out patches has provided strong evidence for the difference between the KirNB and the GIRKs. Before applying Gβγ, we observed the constitutively active Kir (KirNB) only; after the Gβγ application, however, activity of a new channel (GIRK) emerged, and the amplitude of these new channels was certainly larger than the KirNB channel. Further analysis indicated that unlike the GIRK, the activity of the KirNB was not altered by Gβγ. The effect of applying Gαi2 (GDP), which sequesters Gβγ, was tested also. The activity of the KirNB channel was insensitive to Gαi2, whereas the GIRK activity was abolished by Gαi2. Thus, KirNB is functionally different from GIRKs.
All Kir2.0s (classical Kirs) reported thus far have a long open time (on the order of 100 ms). The KirNB channel, having a much shorter open time (1.1 ms), is different from the known classical Kirs. In conclusion, KirNB is different from any of the genetically identified GIRKs or classical Kirs. Although its genetic make-up is yet unknown, the data of its physiological functions indicate that KirNB is a channel that plays important parts in neuronal excitation.
A substantial part of the SP-induced inhibition of KirNB is caused by PKC-induced phosphorylation. On the other hand, the mechanism of the SP-induced GIRK inhibition in brain neurons is unknown, although recent publications have shown that depletion of phosphatidylinositol 4,5-bisphosphate (20–22) or activation of PKC (32, 33) is the main cause for the transmitter-induced GIRK inhibition in heterologous systems.
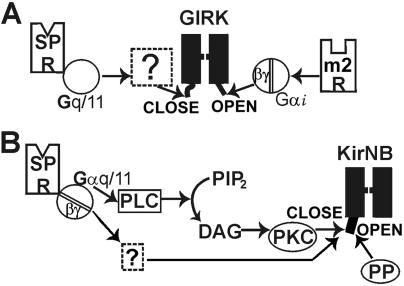
In conclusion, there are two types of Kirs in NB neurons (Fig. 7). One is GIRK, which, with its open time of ≈1 ms and the single-channel conductance of 32–35 pS, is activated by Gβγ. The excitatory transmitter SP inhibits GIRK; the signal transduction of this inhibition, however, is unknown. The other is KirNB, which, with an open time of ≈1 ms and a smaller single-channel conductance (23 pS), is constitutively active and insensitive to Gβγ. SP also inhibits KirNB, and a substantial part of the signal transduction is through PKC-induced phosphorylation. Two important questions remain unanswered. (i) What is the genetic identity of KirNB? (ii) What are the signal transduction pathways leading to the inhibition of GIRK?
Fig 7.
Diagram of the transduction cascade of the SP-induced inhibition of two different types of Kirs in NB neurons. (A) GIRKs are regulated in a dual manner: activated by m2-muscarinic receptor and inhibited by SP receptor (through Gq/11; M.K., J. Collins, T. Kawano, P. Zhao, Q. Zhao, T. Kozasa, S.N., and Y.N., unpublished data). The signal transduction of GIRK inhibition is unknown. (B) The KirNB channel is constitutively active. The SP inhibits the KirNB, and a substantial part of this inhibition is caused by a PKC-mediated phosphorylation (11). The GIRK inhibition is reversed by dephosphorylation. DAG, diacylglycerol; m2 R, m2-muscarinic receptor; PIP2, phosphatidylinositol 4,5-biphosphate; PLC, phospholipase C; SP R, SP receptor; PP, protein phosphatase.
Physiological Functions of the Two Types of Kirs.
What are the functional implications of two different Kirs as effectors for slow excitation? Some brain neurons are receiving excitatory and inhibitory influences almost constantly, and the balance between the two will determine the “tone” of the neurons. Here, GIRKs would play an important role. An example is the mesencephalic dopaminergic neurons, where dopamine, continuously released from the dendrites of the neuron, acts on D2-dopamine receptor on the neuron, causing a continuous state of “autoinhibition” (34, 35). During autoinhibition, GIRKs in dopaminergic neurons would be activated constantly (36). Neurotensin, an endogenous excitatory transmitter for the dopaminergic neurons, can neutralize this GIRK activation (37). When the inhibitory state is neutralized, the neuron would be excited. An analogous situation occurs when the D2 receptor antagonist chlorpromazine is applied; here neuronal excitation (increase in spike frequency) occurs as the result of block of the autoinhibition (34). Thus, elimination of inhibition is sufficient to induce excitation.
It is noted that the dual regulation of the same channel (GIRK) is a mechanism fundamentally different from the “summation” of excitation and inhibition caused by the fast transmitters glutamate and γ-aminobutyric acid (GABA). In the latter case, neutralization (or summation) occurs by using two different channels: the cation channel and the chloride channel. The effects of the two channels are neutralized at the level of membrane potential. By contrast, the mechanism of neutralization involving the GIRK occurs at the level of the same ion channel, which would be more energy-efficient because cancellation of the opposite influences impinging on the same ion channel would not result in expenditure of energy in the form of extra flows of ions.
What then is the role of the constitutively active KirNB channels? Some classes of neurons are often in a quiescent state. Here, the constitutively active KirNB channel plays an important role. Obviously, constant activity of a K+ channel is a necessity for excitation to take place through inhibition of the K channel. A possible advantage of the KirNB channel is that because of its short open time, the channel, depending on the mechanism, could be rapidly activated or inactivated, resulting in a rapid onset of inhibition and excitation.
The two Kir mechanisms for slow excitation may not be confined to NB neurons. It is likely that other brain neurons possess such mechanisms as explained here. Serotonin, through the 5-HT2 receptor, initiates a slow excitation in the nucleus accumbens by inhibiting Kirs (38). Although the transduction mechanism of this slow excitation is unknown, it is possible that a similar mechanism as reported here is involved.
Acknowledgments
We thank Tohru Kozasa (University of Illinois) for the gift of recombinant Gβ1γ2 and Giα2 and Peng Zhao for help in making neuron cultures. This work was supported by National Institutes of Health Grants AG06093 and T32 HL07692, and a Campus Research Board grant from the University of Illinois.
Abbreviations
NB, nucleus basalis
LC, locus coeruleus
Kir, inward rectifier K+ channel
SP, substance P
KirNB, Kir channel in NB
GIRK, G protein-coupled Kir (Kir3.0)
Npo, chance of channel opening
This paper was submitted directly (Track II) to the PNAS office.
Kawano, T., Zhao, P., Nakajima, S. & Nakajima, Y. (2002) Soc. Neurosci. Abstr. 28, Progr. No 645.16 (abstr.).
Bajic, D., Koike, M., Albsoul-Younes, A. M., Nakajima, S. & Nakajima, Y. (2002) Biophys. J. 82, 592 (abstr.).
References
- 1.Mesulam M. M. & Geula, C. (1988) J. Comp. Neurol. 275, 216-240. [DOI] [PubMed] [Google Scholar]
- 2.Everitt B. J. & Robbins, T. W. (1997) Annu. Rev. Psychol. 48, 649-684. [DOI] [PubMed] [Google Scholar]
- 3.Coyle J. T., Price, D. L. & DeLong, M. R. (1983) Science 219, 1184-1190. [DOI] [PubMed] [Google Scholar]
- 4.Chang M. M., Leeman, S. E. & Niall, H. D. (1971) Nat. New Biol. 232, 86-87. [DOI] [PubMed] [Google Scholar]
- 5.Otsuka M., Konishi, S. & Takahashi, T. (1972) Proc. Jpn. Acad. 48, 747-752. [Google Scholar]
- 6.Henry J. L., Krnjevic, K. & Morris, M. E. (1975) Can. J. Physiol. Pharmacol. 53, 423-432. [DOI] [PubMed] [Google Scholar]
- 7.Katayama Y., North, R. A. & Williams, J. T. (1979) Proc. R. Soc. London Ser. B 206, 191-208. [DOI] [PubMed] [Google Scholar]
- 8.Jan L. Y. & Jan, Y. N. (1982) J. Physiol. (London) 327, 219-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanfield P. R., Nakajima, Y. & Yamaguchi, K. (1985) Nature 315, 498-501. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi K., Nakajima, Y., Nakajima, S. & Stanfield, P. R. (1990) J. Physiol. (London) 426, 499-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takano K., Stanfield, P. R., Nakajima, S. & Nakajima, Y. (1995) Neuron 14, 999-1008. [DOI] [PubMed] [Google Scholar]
- 12.Takano K., Yasufuku-Takano, J., Kozasa, T., Singer, W. D., Nakajima, S. & Nakajima, Y. (1996) J. Neurophysiol. 76, 2131-2136. [DOI] [PubMed] [Google Scholar]
- 13.Inoue M., Nakajima, S. & Nakajima, Y. (1988) J. Physiol. (London) 407, 177-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grigg J. J., Kozasa, T., Nakajima, Y. & Nakajima, S. (1996) J. Neurophysiol. 75, 318-328. [DOI] [PubMed] [Google Scholar]
- 15.Kofuji P., Davidson, N. & Lester, H. A. (1995) Proc. Natl. Acad. Sci. USA 92, 6542-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velimirovic B. M., Gordon, E. A., Lim, N. F., Navarro, B. & Clapham, D. E. (1996) FEBS Lett. 379, 31-37. [DOI] [PubMed] [Google Scholar]
- 17.Jelacic T. M., Sims, S. M. & Clapham, D. E. (1999) J. Membr. Biol. 169, 123-129. [DOI] [PubMed] [Google Scholar]
- 18.Koyano K., Velimirovic, B. M., Grigg, J. J., Nakajima, S. & Nakajima, Y. (1993) Eur. J. Neurosci. 5, 1189-1197. [DOI] [PubMed] [Google Scholar]
- 19.Velimirovic B. M., Koyano, K., Nakajima, S. & Nakajima, Y. (1995) Proc. Natl. Acad. Sci. USA 92, 1590-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobrinsky E., Mirshahi, T., Zhang, H., Jin, T. & Logothetis, D. E. (2000) Nat. Cell Biol. 2, 507-514. [DOI] [PubMed] [Google Scholar]
- 21.Cho H., Nam, G. B., Lee, S. H., Earm, Y. E. & Ho, W. K. (2001) J. Biol. Chem. 276, 159-164. [DOI] [PubMed] [Google Scholar]
- 22.Lei Q., Talley, E. M. & Bayliss, D. A. (2001) J. Biol. Chem. 276, 16720-16730. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima Y., Nakajima, S., Obata, K., Carlson, C. G. & Yamaguchi, K. (1985) Proc. Natl. Acad. Sci. USA 82, 6325-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima Y. & Masuko, S. (1996) Neurosci. Res. 26, 195-203. [DOI] [PubMed] [Google Scholar]
- 25.Albsoul-Younes A. M., Sternweis, P. M., Zhao, P., Nakata, H., Nakajima, S., Nakajima, Y. & Kozasa, T. (2001) J. Biol. Chem. 276, 12712-12717. [DOI] [PubMed] [Google Scholar]
- 26.Logothetis D. E., Kurachi, Y., Galper, J., Neer, E. J. & Clapham, D. E. (1987) Nature 325, 321-326. [DOI] [PubMed] [Google Scholar]
- 27.Huang C. L., Slesinger, P. A., Casey, P. J., Jan, Y. N. & Jan, L. Y. (1995) Neuron 15, 1133-1143. [DOI] [PubMed] [Google Scholar]
- 28.Logothetis D. E., Kim, D., Northup, J. K., Neer, E. J. & Clapham, D. E. (1988) Proc. Natl. Acad. Sci. USA 85, 5814-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima Y., Nakajima, S. & Kozasa, T. (1996) FEBS Lett. 390, 217-220. [DOI] [PubMed] [Google Scholar]
- 30.Farkas R. H., Nakajima, S. & Nakajima, Y. (1994) Proc. Natl. Acad. Sci. USA 91, 2853-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey A. I., Edmunds, S. M., Hersch, S. M., Wiley, R. G. & Heilman, C. J. (1995) J. Comp. Neurol. 351, 339-356. [DOI] [PubMed] [Google Scholar]
- 32.Hill J. J. & Peralta, E. G. (2001) J. Biol. Chem. 276, 5505-5510. [DOI] [PubMed] [Google Scholar]
- 33.Leaney J. L., Dekker, L. V. & Tinker, A. (2001) J. Physiol. (London) 534, 367-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunney B. S., Walters, J. R., Roth, R. H. & Aghajanian, G. K. (1973) J. Pharmacol. Exp. Ther. 185, 560-571. [PubMed] [Google Scholar]
- 35.Cheramy A., Leviel, V. & Glowinski, J. (1981) Nature 289, 537-542. [DOI] [PubMed] [Google Scholar]
- 36.Kim K. M., Nakajima, Y. & Nakajima, S. (1995) Neuroscience 69, 1145-1158. [DOI] [PubMed] [Google Scholar]
- 37.Farkas R. H., Chien, P. Y., Nakajima, S. & Nakajima, Y. (1997) Neurosci. Lett. 231, 21-24. [DOI] [PubMed] [Google Scholar]
- 38.North R. A. & Uchimura, N. (1989) J. Physiol. (London) 417, 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colquhoun D. & Sigworth, F. J. (1983) in Single-Channel Recording, eds. Sakmann, B. & Neher, E. (Plenum, New York), pp. 191–263.