Abstract
Movement of an affected hand after stroke is associated with increased activation of ipsilateral motor cortical areas, suggesting that these motor areas in the undamaged hemisphere may adaptively compensate for damaged or disconnected regions. However, this adaptive compensation has not yet been demonstrated directly. Here we used transcranial magnetic stimulation (TMS) to interfere transiently with processing in the ipsilateral primary motor or dorsal premotor cortex (PMd) during finger movements. TMS had a greater effect on patients than controls in a manner that depended on the site, hemisphere, and time of stimulation. In patients with right hemiparesis (but not in healthy controls), TMS applied to PMd early (100 ms) after the cue to move slowed simple reaction-time finger movements by 12% compared with controls. The relative slowing of movements with ipsilateral PMd stimulation in patients correlated with the degree of motor impairment, suggesting that functional recruitment of ipsilateral motor areas was greatest in the more impaired patients. We also used functional magnetic resonance imaging to monitor brain activity in these subjects as they performed the same movements. Slowing of reaction time after premotor cortex TMS in the patients correlated inversely with the relative hemispheric lateralization of functional magnetic resonance imaging activation in PMd. This inverse correlation suggests that the increased activation in ipsilateral cortical motor areas during movements of a paretic hand, shown in this and previous functional imaging studies, represents a functionally relevant, adaptive response to the associated brain injury.
A number of functional brain-imaging studies have demonstrated increased activation of ipsilateral motor areas during movement of the affected limb after stroke (1–7). A popular interpretation of such activation is that areas in the intact hemisphere adaptively compensate for damaged regions (8). However, identification of movement-related activation with functional imaging does not establish the functional significance of this ipsilateral activation. For example, ipsilateral activation could be a correlate of mirror movements (9), reflect potentially maladaptive disinhibition of the intact motor cortex due to reduced transcallosal influences (10), or reflect increased attention to movement (11). An additional possibility is that ipsilateral activation reflects compensation rather than adaptive recovery, i.e., it may reflect use of a different cognitive strategy for movement after stroke, rather than new recruitment of these areas to some functional equivalence with damaged or disconnected contralateral motor regions (8).
The evidence suggesting that adaptive plasticity in the undamaged hemisphere contributes to motor recovery is increasingly compelling (albeit indirect). In a rat model, for example, improved motor function after rehabilitative training following experimental stroke was associated with dendritic growth in the undamaged motor cortex (12). In human imaging studies, increased activation in ipsilateral dorsal premotor cortex (iPMd) during movement of the affected limb has been reported (3, 13) and longitudinal studies show that iPMd activity is present only after some recovery has taken place (14).
Clinical evidence that the undamaged hemisphere may become crucial for recovered movement can be found in reports of patients who have had a second stroke (15). In these cases, patients had recovered movement of the initially affected limb. However, a second stroke in the previously undamaged hemisphere resulted not only in a new contralateral hemiparesis, but also in a reappearance of the original motor deficit in the limb ipsilateral to the second stroke.
However, direct stimulation studies using transcranial magnetic stimulation (TMS) have shown motor-evoked potentials are induced in ipsilateral muscles most commonly in patients with poor recovery (16), and some imaging studies have suggested that ipsilateral activation decreases as recovery occurs (17–19). Thus, it is possible that increased ipsilateral motor cortex involvement is a marker of poor outcome (16), rather than an adaptive response contributing to reduced impairment.
The most direct evidence confirming the role of ipsilateral motor areas in recovery should come from controlled studies of the effects of removing or disrupting these areas. Until now such approaches have been possible only in animal studies, in which candidate regions can be removed or inactivated by cooling or local application of inhibitory drugs (20–22). Studies of recovery of dexterity after M1 lesions in macaque monkeys have suggested that recovery depends principally on preserved function in intact areas in the lesioned hemisphere (21, 22). However, these studies have involved relatively small lesions and are difficult to extrapolate to pathology more typical of human strokes.
The functional significance of ipsilateral motor areas can now be tested in humans by TMS, which can be used to interfere transiently with processing in a specific, chosen cortical area (23). If a task-relevant signal is being processed locally, then interference from the TMS pulse can produce an observable behavioral effect. For example, TMS of M1 after a cue to move can slow reaction times (24).
In addition to providing information on which brain areas are crucial for task performance, single-pulse TMS allows the timing of cortical processing in that area to be determined. For example, the behavioral effects of TMS pulses over M1 and PMd during choice reaction-time tasks depend on pulse timing (25, 26). PMd stimulation slows responses when applied in an early time window (100–140 ms after a cue to move), whereas M1 interference effects occur later (at least 200 ms after the cue) (25). This dissociation is thought to reflect the distinct roles of the two areas, with PMd involved in movement selection and M1 in movement execution. The timing of task disruption by PMd TMS corresponds well to the time when PMd neurons begin to be active in single-unit recording studies of movement selection (27).
Although TMS has been used extensively to map motor representations after stroke by recording muscle responses evoked by cortical stimulation (16), it has not previously been used as a temporary interference technique in stroke to our knowledge. The present study used this approach to investigate the functional importance of ipsilateral motor areas in recovered movements and to extend investigation beyond M1. First, functional MRI (fMRI) was used to quantify activation of ipsilateral motor areas during simple (visually cued index-finger movement) and choice (visually cued random four-finger movement) reaction time (RT) tasks. Second, single-pulse TMS was used to interfere temporarily with processing during the same movements. We then compared the effects of ipsilateral TMS during simple RT movements in stroke patients with those in age-matched controls. Finally, we tested for a relationship between TMS and fMRI measures and between TMS measures and impairment in the patients.
Methods
Subjects.
Sixteen healthy controls and 11 patients after first ischemic left middle cerebral artery territory stroke were tested in accordance with local ethics approval. All subjects were right-handed. Half the controls performed tests with the left, and half with the right hand. Data from controls (age, 40.7 ± 14.0) were analyzed to characterize normal responses to TMS during simple and choice RT tasks. For simple RT only, patients (age, 50.4 ± 11.14) were compared with a subset of five healthy controls who performed tests with the right hand and were adequately age-matched (age, 48.4 ± 14.2). Stroke volume was variable (Table 1), but all patients were in a clinically stable period after first presentations with unilateral hemiparesis. It is likely that the initial hemiparesis was caused by subcortical damage to descending motor tracts. None of the strokes that extended into cortex involved cortex within the volumes of interest defined below for the hand area of M1 (28) or PMd (29). None of the patients had critical stenosis of the proximal internal carotid artery.
Table 1.
Patient details
| Patient | Sex | Age | Volume, cm3 | Time after stroke | Impairment |
|---|---|---|---|---|---|
| 1 | M | 59 | <0.1 | 40 | 10.9 |
| 2 | F | 57 | 8 | 12 | 3.8 |
| 3 | M | 45 | 36 | 24 | −6.4 |
| 4 | M | 55 | 4 | 12 | −0.4 |
| 5 | M | 50 | 12 | 13 | 17.6 |
| 6 | F | 35 | 0.15 | 5 | 12.7 |
| 7 | M | 42 | <0.1 | 8 | −4.1 |
| 8 | M | 53 | <0.1 | 31 | 1.5 |
| 9 | M | 75 | <0.1 | 7 | 5.7 |
| 10 | M | 44 | <0.1 | 4 | 17.9 |
| 11 | M | 46 | <0.1 | 12 | −6.1 |
A hand-impairment measure was calculated for each patient based on reaction times without TMS {impairment = [(A − U)/U] × 100, where A = affected and U = unaffected hand RT}.
fMRI Scanning.
Axial echo-planar volumes (21 × 6 mm slices, TE = 30 ms, TR = 3,000 ms, FOV = 256 × 256, matrix = 64 × 64, voxel size 4 × 4 × 6 mm) and a T1-weighted anatomical image (IR 3D Turbo Flash, 64 × 3 or 1.5 mm axial slices, TR = 30 ms, TE = 5 ms, TI = 500 ms, flip angle = 15°, FOV = 256 × 256, matrix = 256 × 256) were acquired for each subject on a 3T Varian/Siemens MRI system.
Subjects performed 30-s blocks of visually cued RT tasks presented in pseudorandomized order alternating with rest. In separate blocks, simple (always index finger) or choice [cue specifies (at random) which of the four fingers of one hand to press] RT tasks were performed. Subjects practiced the tasks beforehand until they could perform them easily and accurately. All patients could perform the simple RT task well. Some patients were unable to perform the choice RT task, and those who did perform that task did so more slowly than controls (patients, 550 ± 55 ms; age-matched controls, 423 ± 36 ms). For this reason, only data from the simple RT task are reported for patients.
Image Analysis.
We used tools from the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library (FMRIB, Oxford, U.K.; www.fmrib.ox.ac.uk/fsl). The following prestatistics processing was applied: motion correction by using MCFLIRT (30); spatial smoothing by using a Gaussian kernel of FWHM 5 mm; mean-based intensity normalization of all volumes by the same factor; nonlinear high-pass temporal filtering (Gaussian-weighted least squares straight line fitting, with σ = 90 s). Statistical analysis used FILM with local autocorrelation correction (31). Random effects group analyses were performed and group Z statistic images were thresholded by using Z > 3.1, and a cluster significance threshold of P = 0.01, corrected for multiple comparisons (32).
Volumes affected by excessive motion (>10-mm displacements) were discarded. This procedure was necessary for two patients (patients 7 and 9). For one patient (patient 8) excessive motion was present throughout the experiment; therefore, all data from this patient were discarded.
Further analysis of fMRI data focused on individually defined volumes of interest (VOIs) corresponding to sites of ipsilateral TMS and their homologues in the contralateral hemisphere: (i) Primary motor cortex (M1), anterior bank of central sulcus plus posterior half of precentral gyrus superiorly from the dorsal surface of the lateral ventricles; and (ii) dorsal premotor cortex (PMd), anterior half of precentral gyrus and the precentral sulcus superiorly from the dorsal surface of the lateral ventricles. Registration of VOIs to statistical images was performed by using FLIRT (30).
The maximum percent signal change from rest to movement was calculated within each VOI for each task. Effects of task, hemisphere, and brain region were analyzed by using repeated measures general linear models followed up with paired t tests.
Maximum relative signal changes in individual VOI pairs were used to calculate a laterality index [(C − I)/(C + I), where C = contralateral and I = ipsilateral maximum percent signal change]. Paired t tests tested the hypotheses that greater activation would be seen during choice than the simple RT task, that activation would be greater in the contralateral than ipsilateral hemisphere, and that activation would be more lateralized in M1 than PMd.
TMS Testing.
For each subject, one hemisphere was stimulated, and testing was performed on the hand ipsilateral to the stimulated hemisphere. For patients, the nonstroke hemisphere was stimulated and the affected hand was tested. Eight controls were stimulated over the right hemisphere and eight over the left hemisphere.
A figure-of-eight stimulation coil was used to localize M1 (each wing 50 mm in diameter). The coil was connected to a Magstim Rapid Stimulator (Magstim, Camarthenshire, Wales, U.K.) with a maximum output of 2 Tesla. An initial estimate of the scalp position above the hand area of M1 was marked 4 cm lateral and 2 cm anterior to the vertex (Cz). The motor “hot spot” was localized by looking for visible finger movement of the contralateral hand in response to TMS of points around this mark (25, 26).
Two stimulation sites were marked relative to this hot spot: a primary sensorimotor site (M1) 1 cm posterior to the hot spot and a dorsal premotor site (PMd) 2 cm anterior and 1 cm medial to the hot spot. Previous studies have shown that TMS at these two sites leads to dissociable effects on movement selection and execution (25, 26).
For the remainder of the experiment a slightly larger figure-of-eight coil was used (70-mm wing diameter) for both sites. Use of this larger coil increased the likelihood that each of the crucial regions would be stimulated as intended. Before this final stage of testing, motor thresholds were remeasured at M1 with the larger coil. We reconfirmed that stimulation over the PMd site at the same intensity did not evoke visible movement with the larger coil. Stimulation was applied at 120% of M1 threshold for most subjects (55–74% of maximum stimulator output). For three subjects (two controls, one patient) with apparently high thresholds, stimulation was applied at 115% of threshold to minimize discomfort (76–85% of maximum stimulator output).
All controls performed the simple and choice RT tasks they had performed previously during fMRI. All patients performed the simple RT task. Tasks were performed in blocks of 60 trials, twice with M1-directed TMS and twice with PMd-directed TMS. Subjects practiced each task without TMS first. Patients practiced first with the unaffected and then with the affected hand. On TMS blocks stimulation was randomly applied on half of all trials at one of five stimulation times: 50, 100, 150, 200, and 250 ms after the movement cue. Data were analyzed for all five time points in the choice RT task, but only the first three time points were analyzed in the more quickly performed simple RT task.
Frameless Stereotactic MRI-Guided Localization of TMS Sites.
A Polaris IR tracking device (Northern Digital, Waterloo, ON, Canada) was used to detect reflective probes attached to landmarks (nose tip and bridge, left and right tragus) and reference points on the subject's head and the TMS coil. BRAINSIGHT software (Rogue Research, Montreal) was used to coregister the subject's head with their MRI scan. This system was used to confirm individually the localization of stimulation sites with respect to anatomical landmarks on each subject's MRI scan (33).
Analysis of TMS Reaction Times.
Median correct RTs were found for each condition with and without TMS. The relative change in RT from the no-TMS baseline was calculated for each time point, condition, and stimulation site. Effects of stroke, task, stimulation time, and site were tested with repeated measures general linear models and paired t tests. Pearson's correlation coefficients were calculated to assess correlations between fMRI, TMS, and impairment measures in patients.
Results
Experiment 1: Simple and Choice RT Tasks in Controls.
Movement-related fMRI activation.
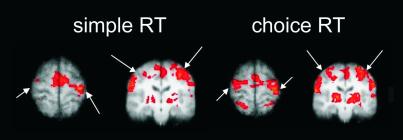
Controls activated the expected network of sensorimotor regions during finger movements (Fig. 1). Activation was detected in the ipsilateral hemisphere for both tasks.
Fig 1.
In controls, choice RT tasks (Right) produced more overall, and more bilateral fMRI activation than simple RT tasks (Left). Activation from left- and right-hand groups have been combined by rotating the data for left-hand movement about the midline. The left hemisphere is on the right-hand side of the images. Images are thresholded at Z > 3.1; cluster extent threshold of P < 0.01. Arrows indicate position of the central sulcus.
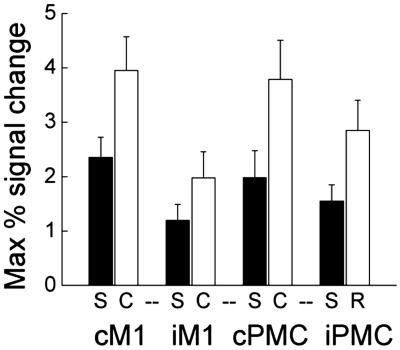
The maximum percent signal changes within the chosen VOIs were calculated (Fig. 2). Greater activation was seen during choice RT than with simple RT in all VOIs. Overall, activation was greater in the hemisphere contralateral to the hand moved. The difference between contra- and ipsilateral activation was significant for M1, but not for PMd (Fig. 2). PMd activation had a lower laterality index (i.e., activation was less lateralized to the contralateral hemisphere) than M1 (F = 5.66, P = 0.032) for both tasks (simple, t = 5.00, P < 0.001; choice, t = 3.86, P = 0.002).
Fig 2.
Mean values for fMRI signal change within VOIs for healthy controls. More activity occurred during choice (white bars) than during simple (black bars) RT tasks (F = 69.562, P < 0.001) for all VOIs (CM1: t = 6.2, P < 0.001; IM1: t = 5.2, P < 0.001; cPMd: t = −7.0, P < 0.001; iPMd: t = −5.6, P < 0.001). More activity occurred contralateral than ipsilateral to the hand moved (F = 10.519, P = 0.006). This laterality difference was significant for M1 (simple: t = 3.10, P = 0.007; choice: t = 4.64, P < 0.001) but not PMd (simple: NS; choice: t = 1.83, P = 0.088). Error bars represent standard errors.
Effect of TMS on reaction times.
We wished to test the functional significance of the ipsilateral activation detected with fMRI. We did this in separate studies of the same subjects by applying TMS to interfere temporarily with processing in ipsilateral M1 and PMd during identical simple or choice RT tasks.
Mean reaction times without TMS were significantly slower for the choice than the simple RT task (simple, 224.9 ± 42.4 ms; choice, 403.6 ± 28.3 ms; t = 5.84, P < 0.001). The relative changes in RT from this baseline induced by TMS at selected times after the cue to move were calculated (Fig. 3). To test for differences in TMS effects with different movements, stimulation sites and times, the relative changes in RT were analyzed in a repeated measures general linear models. Consistent with the finding of increased ipsilateral fMRI activation during the choice RT task, ipsilateral TMS slowed responses more during the choice than with the simple RT task (F = 17.26, P < 0.001). However, the differential effects of TMS on the two movement tasks depended also on the site and time of stimulation (task × time, F = 7.49, P = 0.002; task × site, F = 10.30, P = 0.006).
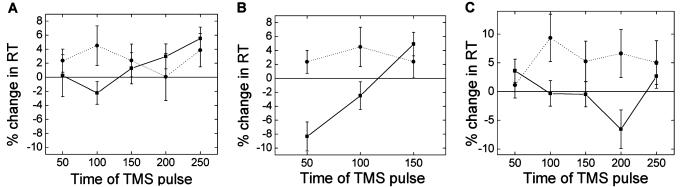
Fig 3.
Behavioral effects of TMS for the healthy control group. (A) During choice RT task an early time period exists during which ipsilateral TMS has an effect when applied over premotor (dotted line) but not primary motor (solid line) cortex. (B) Early involvement of iPMd is specific to choice (dotted line) rather than simple (solid line) RT tasks. (C) Early involvement of iPMd in choice RT was greater for left (dotted) than right (solid) hemisphere stimulation. Error bars represent standard errors.
A specific role for ipsilateral PMd (iPMd) [and not ipsilateral M1 (iM1)] early in the choice RT task can be seen in Fig. 3A; TMS over iPMd slows responses more than TMS over iM1 at 100 ms in the choice RT task (t = −2.31, P = 0.035, Fig. 3A). Early involvement of iPMd is specific to the choice (and not simple) RT task; TMS over iPMd has a greater effect on the choice than the simple RT task both at 50 ms (t = −3.88, P = 0.001) and at 100 ms (t = −2.58, P = 0.021) (Fig. 3B). The speeding effects of TMS at certain time points (e.g., M1 TMS at 50 ms during the choice RT task) is probably due to multisensory facilitation effects (e.g., the auditory and tactile stimulation that occur with a TMS pulse speeds responses to the visual cue) that often occur when TMS is applied over an area when it is not specifically involved in a task (23).
We studied both right- and left-hand movements. Ipsilateral TMS slowed responses more when applied over the left than the right hemisphere (F = 9.84, P = 0.007). The dependence of TMS effects on the time and site of stimulation also varied between left- and right-hand movements (site × time × hand, F = 4.56, P = 0.019). Slowing effects of early (100 ms) TMS of iPMd during the choice RT task were only seen for the left hemisphere (Fig. 3C).
Experiment 2: Comparison of Patients and Age-Matched Controls During a Simple RT Task.
Movement-related fMRI activation.
Like controls, patients activated a network of sensorimotor areas during hand movement. Analysis of signal changes within VOIs during the simple RT task showed that large variations occurred in the magnitude, extent, and laterality of fMRI activations among patients (who were heterogeneous for lesion volume and for residual functional impairment) (Fig. 4). No significant group differences between patients and controls occurred in any VOIs.
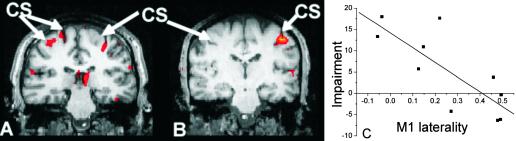
Fig 4.
Variability occurred in the relative lateralization of fMRI activation in patients. (A and B) Representative activation maps for a simple RT task versus rest for two individual patients. Bilateral motor cortex activation was most common in more impaired patients (e.g., A, illustrating results from a patient with impairment score of 17.9). Predominantly contralateral activation (i.e., similar to the control pattern) was most common in less impaired patients (e.g., B, from a patient with impairment score of −6.2). We found a correlation between impairment and lateralization of fMRI activity (C) fMRI data are thresholded at Z >3.1, and a cluster extent threshold of P < 0.01.
We tested for a relationship between fMRI measures and hand impairment. Relatively increased ipsilateral activation, reflected by lower laterality indices, was most common in poorly recovered patients; we found a negative correlation between impairment and fMRI laterality in M1 (r = −0.79, P = 0.007, Fig. 4) and a trend for a correlation with laterality in PMd (r = −0.55, P = 0.1).
We wished to test the functional significance of ipsilateral activation in patients and to explore whether the pattern of TMS-induced disruption of a simple RT task differed between patients and controls.
Effect of TMS on reaction times.
Without TMS, reaction times for the simple RT task in patients (240.7 ± 48.3 ms) were similar to age-matched controls (228.9 ± 23.5 ms).
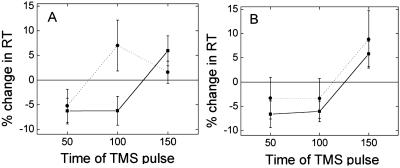
The relative change in RT with ipsilateral TMS applied to either M1 or PMd varied with the time of stimulation relative to the cue (M1: F = 9.91, P = 0.001; PMd: F = 5.57, P = 0.01; Fig. 5). With TMS of iPMd the effects of TMS varied significantly between patients and healthy controls; a significant interaction occurred between stimulation time and group (F = 4.84, P = 0.016), suggesting that differences between patients and controls were stimulation-time-dependent. No interactions were seen with iM1 TMS. The effects of iPMd TMS were greater for patients than for controls when applied 100 ms after the cue to move (Fig. 5). The results suggest that at least some of the patients are recruiting iPMd early after the cue to move.
Fig 5.
(A) TMS over iPMd during a simple RT task had distinct effects in patients (dotted line) and controls (solid line). Pulses at 100 ms slowed patients but not controls. This early slowing effect of iPMd TMS was only seen in controls during a choice RT task (see Fig. 3). (B) No clear differences between patients and controls were seen with TMS over iMC. Error bars represent standard errors.
This pattern of iPMd involvement in patients is similar to the pattern seen in control subjects during a choice RT task (Fig. 3 A and B). However, in control subjects we found significantly greater effects of ipsilateral TMS over the left hemisphere than over the right hemisphere. The age-matched controls in experiment 2 were tested with the right hemisphere and right hand (for comparison with the patients with left-hemisphere stroke). Early TMS of right PMd does not affect choice RT with the right hand in healthy controls (Fig. 3C).
Correlations between TMS interference and fMRI or hand impairment.
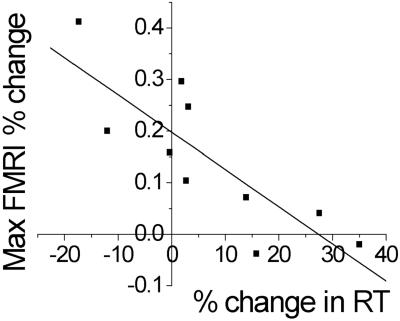
We then tested the extent to which involvement of iPMd at 100 ms reflected patterns of activation present in the fMRI data for patients. We found a negative correlation between the magnitude of the 100-ms iPMd TMS effect and fMRI laterality index in PMd (r = −0.82, P = 0.004; Fig. 6), which suggests that the laterality of fMRI signal changes reflect functionally significant activity.
Fig 6.
In patients significant correlation occurred between TMS and fMRI measures. Patients with a low PMd fMRI laterality index (i.e., relatively bilateral) during a simple RT task also showed a large slowing effect of 100-ms iPMd TMS during the same task. The upper confidence limit (one-tailed, P < 0.05) for percent change in RT with TMS for age-matched controls was −0.006%. Seven of 10 patients fall outside this limit.
Finally, we tested whether the magnitude of TMS effects was related to the degree of hand impairment. We found a positive correlation between the effect of iPMd TMS at 100 ms and a measure of finger-movement impairment in patients that was close to significance (r = 0.62, P = 0.057).
Confirmation of targets of TMS.
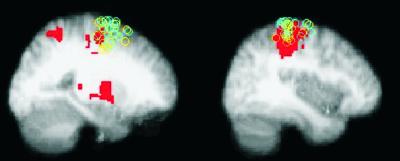
TMS trajectories for patients and controls overlapped (Fig. 7). M1 trajectories met the cortical surface around the central sulcus, and PMd trajectories met the cortical surface around the anterior precentral gyrus or superior precentral sulcus (Fig. 7).
Fig 7.
MRI guided confirmation of sites of TMS for patients (blue) and controls (yellow) in standard space. TMS targets for all subjects overlaid on the control fMRI maps (Z > 3.1, P < 0.01). (Left) PMd site; (Right) M1 site. Sagittal slices are at the mean × coordinate for each site (PMd, x = 24; M1, x = 40).
Discussion
fMRI scanning of patients after stroke demonstrated that patterns of brain activity alter after injury; brain activation during simple finger movements is more bilateral in more severely impaired patients. However, it is uncertain whether the ipsilateral, motor-related activity is behaviorally significant. We therefore used TMS to test the functional significance of this activation specifically.
Interference with ipsilateral motor areas by TMS slowed movements in both healthy controls and patients, but interference in patients occurred in a manner distinct from that seen in the healthy controls. The differential effect of TMS in patients and controls depended on pulse location and timing. TMS over the right PMd slowed right index finger movements in right hemiparetic patients, but not in age-matched controls when applied 100 ms after the cue to move (Fig. 5A), which suggests that the ipsilateral motor cortical activation observed in patients after strokes causing hemiparesis reflects functionally significant involvement of this cortex in the motor task. The magnitude of the 100-ms iPMd TMS slowing effect in patients correlated with the laterality of PMd fMRI activation during the simple RT task, so fMRI activity lateralization after stroke reflects the relative magnitude of this adaptive activity in ipsilateral cortex. The magnitude of the 100-ms iPMd TMS slowing effect in patients also correlated with impairment, suggesting that poorly recovered patients depend more on activity in iPMd to perform a simple RT task.
The TMS and fMRI methods used here provide information on very different temporal scales. Although dissociable effects can be found with TMS pulses 50 ms apart, the changes in BOLD signal detected here will reflect activity over the whole of a 30-s task block. Although both healthy controls and patients may have fMRI activity in iPMd, TMS allows a clear distinction to be made between these activations for the two groups. The timing of TMS interference suggests that in healthy subjects the role of iPMd in this task is limited to the late stages of a trial, whereas in stroke patients iPMd is additionally involved in the early stages of a trial.
In healthy controls, involvement of ipsilateral motor areas is greater with more complex movements (34, 35). The increased involvement of ipsilateral premotor cortex in simple movements of an impaired limb could just reflect the relative difficulty of such movements. The timing of the interference effects of iPMd during the simple task in patients is similar to that observed for the more complex, choice RT task in healthy controls here (Fig. 3A) and in previous studies of a similar task (25), also suggesting that simple movements after stroke may involve a spatial and temporal pattern of motor cortical activity similar to that associated with more complex movements in healthy controls. Nonetheless, the results from patients imply a quantitative change in the functional relationships between brain activity in iPMd and behavior after injury.
In healthy controls early (100 ms) involvement of iPMd in choice RT was apparent with TMS of the left hemisphere only [Fig. 3C, reflecting a greater role for the left hemisphere in ipsilateral movements as reported (25, 36, 37)]. Whereas in patients with left hemisphere stroke effects on the simple task were seen with right PMd stimulation, suggesting that this cortex plays a role in simple movements of the right (affected) hand that was not observed in healthy controls for any of the right-hand movements tested here. Thus, a qualitative change also occurs in the relationships between brain activity in iPMd and behavior after injury.
Because hemodynamic and electrical changes can be detected in adjacent and interconnected areas after a TMS pulse (33), it is important to consider whether the observed effects could be mediated by transcallosal stimulation of cPMd (38), which seems unlikely. If the effects of ipsilateral stimulation were largely mediated by transcallosal influence on contralateral areas, then direct contralateral stimulation should produce similar effects. However, contralateral stimulation produces qualitatively different patterns of disruption to ipsilateral stimulation (25). In addition, in the current study we found a negative correlation between the laterality of fMRI PMd activation and the size of the PMd TMS slowing effects; effects of iPMd TMS were greatest in patients who showed relatively increased ipsilateral (or decreased contralateral) activation of PMd.
Our TMS results suggest that it is the premotor rather than the primary motor cortex of the ipsilateral hemisphere that is differentially more involved in patients relative to controls for simple movements. Consistent with this finding, previous imaging studies have reported increased activation in iPMd during movement of the affected limb (3, 13), and the present study has shown that such activations tend to be found in more impaired patients. This finding could reflect relatively increased recruitment of uncrossed corticospinal projections from the PMd in patients compared with controls during this simple task, as has been reported for the damaged hemisphere in acute stroke (39). Although the majority of corticospinal projections originate in M1, a substantial proportion come from other motor areas (40). In addition, whereas 70–90% of pyramidal fibers decussate into the lateral corticospinal tract, 10–30% are uncrossed and descend as the ventral corticospinal tract (41). PMd has prominent bilateral connections to the spinal cord (42). However, the ipsilateral connections of PMd are with ventromedial spinal areas that are less concerned with distal movement. The pattern of spinal connectivity from PMd therefore may constrain the degree of recovery possible. The more impaired patients, in whom iPMd seemed to be most important for movement, were able to make the simple movements needed for the simple RT task, but were unable to make the individual finger movements required for the choice RT task. Projections of nonprimary motor areas to spinal motor neurons have a pattern distinct from those of M1 (43), which also may limit the extent and manner in which PMd can contribute to recovery. Further experiments are needed to establish the anatomical route by which iPMd influences the spinal cord in these patients
Involvement of iPMd was greatest in the more impaired patients. Therefore, the present results cannot be interpreted as showing that the undamaged premotor cortex is functionally substituting for the injured contralateral motor system in a complete and simple way (8). What seems clear, however, is that increased activity in iPMd is not maladaptive (16), because the effects of TMS disruption have demonstrated that iPMd activity is functionally significant. iPMd behaves as if it mediates partial adaptive compensation for injured motor cortex after stroke. Although greater injury produces greater impairment, it also provokes a greater adaptive response. Although increased use of iPMd does not enable complete recovery in the most impaired patients, it is likely that it enables greater recovery than would have been possible otherwise.
Acknowledgments
We thank Helen Dawes, Joe Devlin, and Donna Lloyd for help with patient testing, Derick Wade for additional help with patient recruitment, and John Stein for useful comments on the manuscript. We acknowledge the generous support of the Wellcome Trust (H.J.-B.), the Royal Society (M.F.S.R.), and the Medical Research Council (P.M.M. and M.F.S.R.).
Abbreviations
PMd, dorsal premotor cortex
TMS, transcranial magnetic stimulation
fMRI, functional MRI
VOIs, volumes of interest
RT, reaction time
iPMd, ipsilateral PMd
References
- 1.Cramer S. C., Nelles, G., Benson, R. R., Kaplan, J. D., Parker, R. A., Kwong, K. K., Kennedy, D. N., Finklestein, S. P. & Rosen, B. R. (1997) Stroke 28, 2518-2527. [DOI] [PubMed] [Google Scholar]
- 2.Cuadrado M. L., Egido, J. A., Gonzalez-Gutierrez, J. L. & Varela-De-Seijas, E. (1999) Cerebrovasc. Dis. 9, 337-344. [DOI] [PubMed] [Google Scholar]
- 3.Weiller C., Chollet, F., Friston, K. J., Wise, R. J. & Frackowiak, R. S. (1992) Ann. Neurol. 31, 463-472. [DOI] [PubMed] [Google Scholar]
- 4.Chollet F., DiPiero, V., Wise, R. J., Brooks, D. J., Dolan, R. J. & Frackowiak, R. S. (1991) Ann. Neurol. 29, 63-71. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y., D'Olhaberriague, L., Vikingstad, E. M., Levine, S. R. & Welch, K. M. (1998) Stroke 29, 112-122. [DOI] [PubMed] [Google Scholar]
- 6.Caramia M. D., Iani, C. & Bernardi, G. (1996) NeuroReport 7, 1756-1760. [DOI] [PubMed] [Google Scholar]
- 7.Honda M., Nagamine, T., Fukuyama, H., Yonekura, Y., Kimura, J. & Shibasaki, H. (1997) J. Neurol. Sci. 146, 117-126. [DOI] [PubMed] [Google Scholar]
- 8.Fries W., Danek, A., Scheidtmann, K. & Hamburger, C. (1993) Brain 116, 369-382. [DOI] [PubMed] [Google Scholar]
- 9.Weiller C., Ramsay, S. C., Wise, R. J., Friston, K. J. & Frackowiak, R. S. (1993) Ann. Neurol. 33, 181-189. [DOI] [PubMed] [Google Scholar]
- 10.Meyer B. U., Roricht, S., von Einsiedel, G., Kruggel, F. & Weindl, A. (1995) Brain 118, 429-440. [DOI] [PubMed] [Google Scholar]
- 11.Johansen-Berg H. & Matthews, M. (2002) Exp. Brain Res. 142, 13-24. [DOI] [PubMed] [Google Scholar]
- 12.Biernaskie J. & Corbett, D. (2001) J. Neurosci. 21, 5272-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seitz R. J., Hoflich, P., Binkofski, F., Tellmann, L., Herzog, H. & Freund, H. J. (1998) Arch. Neurol. 55, 1081-1088. [DOI] [PubMed] [Google Scholar]
- 14.Nelles G., Spiekramann, G., Jueptner, M., Leonhardt, G., Muller, S., Gerhard, H. & Diener, H. C. (1999) Ann. Neurol. 46, 901-909. [DOI] [PubMed] [Google Scholar]
- 15.Fisher C. M. (1992) Can. J. Neurol. Sci. 19, 57-63. [PubMed] [Google Scholar]
- 16.Turton A., Wroe, S., Trepte, N., Fraser, C. & Lemon, R. N. (1996) Electroencephalogr. Clin. Neurophysiol. 101, 316-328. [DOI] [PubMed] [Google Scholar]
- 17.Marshall R. S., Perera, G. M., Lazar, R. M., Krakauer, J. W., Constantine, R. C. & DeLaPaz, R. L. (2000) Stroke 31, 656-661. [DOI] [PubMed] [Google Scholar]
- 18.Carey J. R., Kimberley, T. J., Lewis, S. M., Auerbach, E. J., Dorsey, L., Rundquist, P. & Ugurbil, K. (2002) Brain 125, 773-788. [DOI] [PubMed] [Google Scholar]
- 19.Feydy A., Carlier, R., Roby-Brami, A., Bussel, B., Cazalis, F., Pierot, L., Burnod, Y. & Maier, M. A. (2002) Stroke 33, 1610-1617. [DOI] [PubMed] [Google Scholar]
- 20.Bornschlegl M. & Asanuma, H. (1987) Brain Res. 437, 121-130. [DOI] [PubMed] [Google Scholar]
- 21.Rouiller E. M., Yu, X. H., Moret, V., Tempini, A., Wiesendanger, M. & Liang, F. (1998) Eur. J. Neurosci. 10, 729-740. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y. & Rouiller, E. M. (1999) Exp. Brain Res. 128, 149-159. [DOI] [PubMed] [Google Scholar]
- 23.Walsh V. & Rushworth, M. (1999) Neuropsychologia 37, 125-135. [PubMed] [Google Scholar]
- 24.Day B. L., Dressler, D., Maertens de Noordhout, A., Marsden, C. D., Nakashima, K., Rothwell, J. C. & Thompson, P. D. (1989) J. Physiol. 412, 449-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schluter N. D., Rushworth, M. F., Passingham, R. E. & Mills, K. R. (1998) Brain 121, 785-799. [DOI] [PubMed] [Google Scholar]
- 26.Schluter N. D., Rushworth, M. F., Mills, K. R. & Passingham, R. E. (1999) Neuropsychologia 37, 233-243. [DOI] [PubMed] [Google Scholar]
- 27.di Pellegrino G. & Wise, S. P. (1991) Brain 114, 951-978. [DOI] [PubMed] [Google Scholar]
- 28.Yousry T. A., Schmid, U. D., Alkadhi, H., Schmidt, D., Peraud, A., Buettner, A. & Winkler, P. (1997) Brain 120, 141-157. [DOI] [PubMed] [Google Scholar]
- 29.Rizzolatti G., Luppino, G. & Matelli, M. (1998) Electroencephalogr. Clin. Neurophysiol. 106, 283-296. [DOI] [PubMed] [Google Scholar]
- 30.Jenkinson M. & Smith, S. (2001) Med. Image Anal. 5, 143-156. [DOI] [PubMed] [Google Scholar]
- 31.Woolrich M. W., Ripley, B. D., Brady, M. & Smith, S. M. (2001) NeuroImage 14, 1370-1386. [DOI] [PubMed] [Google Scholar]
- 32.Forman S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A. & Noll, D. C. (1995) Magn. Reson. Med. 33, 636-647. [DOI] [PubMed] [Google Scholar]
- 33.Paus T., Jech, R., Thompson, C., Comeau, R., Peters, T. & Evans, A. L. (1997) J. Neurosci. 17, 3178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao S. M., Binder, J. R., Bandettini, P. A., Hammeke, T. A., Yetkin, F. Z., Jesmanowicz, A., Lisk, L. M., Morris, G. L., Mueller, W. M., Estkowski, L. D., et al. (1993) Neurology 43, 2311-2318. [DOI] [PubMed] [Google Scholar]
- 35.Chen R., Gerloff, C., Hallett, M. & Cohen, L. G. (1997) Ann. Neurol. 41, 247-254. [DOI] [PubMed] [Google Scholar]
- 36.Schluter N. D., Krams, M., Rushworth, M. F. & Passingham, R. E. (2001) Neuropsychologia 39, 105-113. [DOI] [PubMed] [Google Scholar]
- 37.Chen R., Cohen, L. G. & Hallett, M. (1997) Can. J. Neurol. Sci. 24, 284-291. [DOI] [PubMed] [Google Scholar]
- 38.Ilmoniemi R. J., Virtanen, J., Ruohonen, J., Karhu, J., Aronen, H. J., Naatanen, R. & Katila, T. (1997) NeuroReport 8, 3537-3540. [DOI] [PubMed] [Google Scholar]
- 39.Alagona G., Delvaux, V., Gerard, P., De Pasqual, V., Pennisi, G., Delwaide, P. J., Nicoletti, F. & Maertens de Noordhout, A. (2001) Stroke 32, 1304-1309. [DOI] [PubMed] [Google Scholar]
- 40.Dum R. P. & Strick, P. L. (1991) J. Neurosci. 11, 667-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan P. W. & Smith, M. C. (1973) Brain 96, 471-494. [DOI] [PubMed] [Google Scholar]
- 42.Kuypers H. G. & Brinkman, J. (1970) Brain Res. 24, 29-48. [DOI] [PubMed] [Google Scholar]
- 43.Maier M. A., Armand, J., Kirkwood, P. A., Yang, H. W., Davis, J. N. & Lemon, R. N. (2002) Cereb. Cortex 12, 281-296. [DOI] [PubMed] [Google Scholar]