Abstract
Dopamine is thought to exert a negative control on lactotrop cell proliferation and prolactin production. Indeed, mice lacking the D2 receptor develop pituitary tumors of lactotrop origin. Because lactotrops express two isoforms of D2R, D2L, and D2S, in a specific ratio, we decided to explore the physiological importance of their relative abundance in vivo. Thus, we generated transgenic animals overexpressing either D2L or D2S in lactotrops. Increased expression of D2S, but not of D2L, leads to mitogen-activated protein kinase (MAPK) induction, which results in pituitary hypoplasia. On the other hand, levels of phosphorylated MAPKs are drastically reduced in pituitary tumors generated by the absence of D2-dependent signaling. These results underline a critical role of D2-mediated MAPK activation in lactotrop proliferation. Furthermore, whereas D2S overexpression results to a drastic reduction of prolactin, D2L overexpression elevates it. Our findings underscore a different role of the two D2R isoforms in the pituitary gland physiology.
Keywords: DA, D2L and D2S receptors, pituitary, MAPK
Activation of dopamine D2 receptor (D2R) regulates several physiological functions in the central nervous system as well as in the pituitary gland (1–3). In the pituitary, the signal transduction activated by D2 receptors negatively regulates prolactin (PRL) synthesis and release (4, 5). Analyses of D2R null mice have illustrated a control on the proliferative rate of lactotrops by D2R. In the absence of D2R signaling pituitary tumors arise of lactotrop origin (prolactinomas) in 100% of aged females and only very rarely in males (2, 6, 7). In humans, prolactinomas might regress on treatment with dopaminergic agonists such as bromocriptine and are generally more common in women than in men (8, 9). Thus, similar D2R-mediated signaling seems to exist at the pituitary level in humans and mice. It is presently unclear through which pathway the activation of D2R is able to control lactotrop proliferation.
D2Rs exist into two molecularly distinct isoforms, D2L and D2S. Both isoforms, generated by alternative splicing from the same gene, have similar pharmacological and biochemical profiles in vitro, despite the presence of an additional 29 amino acids in the D2L isoform (10–13). This additional segment is located in the third intracellular loop of the putative receptor structure, a region involved in the coupling to the G proteins. In vitro studies suggest that the two isoforms might activate different signaling pathways in vivo (14). Analyses with genetically modified mice have recently supported the hypothesis that D2L and D2S might have different functions in vivo (15, 16).
The two isoforms are produced in a well-defined D2L/D2S ratio. The D2L isoform is always more abundant than D2S. This ratio is respected in the pituitary and in the brain with the exception of mesencephalic regions where it is inverted (13, 17). The physiological role of the ratio of the two different isoforms is still unknown. In this study, we have addressed this point by analyzing the consequence of altering the expression of D2L and D2S on lactotrops physiology, both at the cellular and biochemical levels.
We have generated transgenic animals overexpressing either isoform of the D2R in the lactotrops and analyzed how D2-mediated signaling affects lactotrop proliferation both in vivo and in vitro. We show that stimulation of D2R leads to the activation of p44/42 extracellular-regulated kinase (ERKs) of the mitogen-activated protein kinase (MAPK) pathway in the pituitary. We show that D2L and D2S differentially activate this pathway. ERKs activation is correlated with an inhibition of lactotrop proliferation both in vitro and in vivo. Indeed, the development of prolactinomas found in aged D2R-null female mice is accompanied by a concomitant decrease in the phosphorylation of these kinases. Thus, these studies clearly show an antagonistic effect of ERK activation mediated by D2R on lactotrop proliferation.
Materials and Methods
Generation of D2L and D2S Transgenic Lines.
Transgenic lines were generated by using a 3,033-bp fragment (BamHI-HindIII) of the PRL gene promoter (−3,000/+33) (18). The fragment was ligated to D2L or D2S cDNA (13), respectively, of 2,261 and 2,174 bp. Finally a fragment of 847 bp (BamHI-BglII) corresponding to the simian virus 40 (SV40) late polyadenylation site (19) was cloned at the 3′ of PRL-D2L or PRL-D2S (Fig. 1). Both constructs were injected in C57BL6/SJL fertilized oocytes. The transgenic animals used in this study are in a pure C57/BL6 background. Transgenics were identified by Southern blot analysis of tail biopsies of genomic DNA digested with PstI. Blots were hybridized with a random primed 32P-labeled probe corresponding to the SV40 late polyadenylation site. The number of copies of the transgene was evaluated by comparing dot-blot analysis of transgenic DNA with injected fragment (20). D2R knockout (1) had a mixed 129Sv × C57BL/6 genetic background, with a 75% contribution of C57BL/6. Animals were housed in a 12-h light/dark cycle with food and water available ad libitum. All of the experiments were performed with female mice.
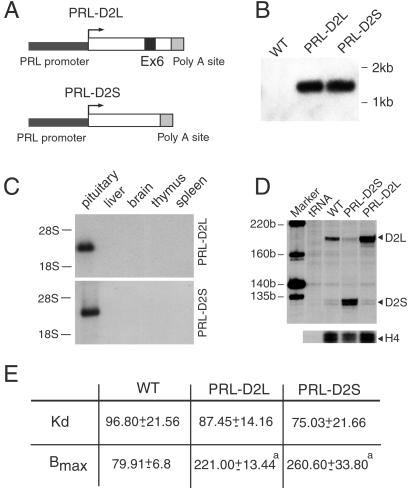
Fig 1.
Analyses of PRL-D2L and PRL-D2S transgenic mice. (A) Schematic representation of the chimeric constructs used to generate the PRL-D2L and PRL-D2S transgenic lines. Exon 6 (black box) is absent in the D2S cDNA. The 3-kb promoter region of the rat PRL gene is indicated in black. The polyadenylylation site of the SV40 large T antigen (gray box) has been added at the 3′ of each construct. (B) Southern blot analysis of genomic DNA from tail biopsies from WT, PRL-D2L, and PRL-D2S mice. Genomic DNA was digested by PstI, blotted, and hybridized by using the SV40 poly(A) sequence as probe. (C) Two micrograms of total RNA from the pituitary gland and 10 μg of all of the other tissues of animals of each genotype were used for Northern blot analysis. The filter was hybridized with a 32P-labeled probe corresponding to the SV40 poly(A) sequence. (D) RNase protections were performed by using 5 μg of total RNA hybridized with a mouse D2R specific 32P-labeled probe. The size of protected fragments was 202 bp (D2L) and 131 bp (D2S) as indicated. A histone H4 probe was used as an internal control. (E) [3H]Spiperone binding on membrane extracts from pituitaries of WT, PRL-D2L, and PRL-D2S mice. Nonspecific binding was determined in the presence of (+)-butaclamol (1 μM). The affinity (Kd) and the maximal number of binding sites (Bmax) were calculated on Scatchard's transformation of saturation curves. The Kd is expressed in picomolar and the Bmax in femtomoles of D2 receptor per mg protein. Values are mean ± SEM of three different experiments performed in duplicates. a = P < 0.001 (***) versus wild-type mice (Student's t test).
RNA Preparation, Northern Blot Analysis, RNase Protection, and Binding Assay.
Total RNA from different organs was prepared by the guanidinium-thiocyanate method (44). Northern blot analysis was performed by using a random primed 32P-probe of the SV40 polyadenylation site. Two micrograms of total RNA from pituitary tissue and 10 μg from all of the other tissues were run on formaldehyde/agarose gel in 10 mM sodium phosphate buffer and transferred to Hybond N+ (Amersham Pharmacia). Membranes were hybridized overnight at 42°C in 50% formamide, washed 10 min two times at room temperature and two times at 60°C under high stringent conditions (2× SSC/0.1% SDS), and then exposed to Kodak X-Omat films. RNase protections assays and PCR were performed as described (15, 21). Binding assays were performed as described (15); in brief 15 μg of pituitary membranes were used for ligand-binding assays with [3H]spiperone (specific activity, 114 Ci mmol−1; Amersham Pharmacia). Concentrations of [3H]spiperone of 10–600 pM were used and nonspecific binding was determined in presence of 1 μM cold (+)-butaclamol. Binding data were analyzed with the EBDA-LIGAND program (Elsevier-BIOSOFT, Amsterdam).
In Situ Hybridization and Immunofluorescence.
Pituitary cryostat sections (10 μm) were prepared from 4-month-old WT and PRL-D2L or PRL-D2S female mice. In situ hybridization was performed as described (2). After antisense RNA hybridization and washing, the cryostat sections were dipped in Kodak NTB-2 autoradiography emulsion and exposed for 24 h. Nuclei were counterstained by using toluidine blue. The specificity of in situ hybridization results was confirmed by absence of detectable signals with the sense riboprobes.
For immunofluorescence pituitary, cryostat sections were postfixed in formalin (Sigma) for 15 min, preincubated 1 h in 5% normal goat serum and 0.05% Tween 20 in PBS 1×, followed by incubation with rabbit anti-PRL antibody (1:2,000) (Euromedex Mundolsheim, France) at 4°C overnight. Slides were then incubated 1 h with goat anti-rabbit conjugated with Cy3 (1:1,000) (Jackson ImmunoResearch). Controls for antibody specificity were obtained by incubation of sections only with the secondary antibody. The number of lactotrops was estimated by counting the number of PRL-positive cells (Cy3, red staining) over the total number of nuclei (4′,6-diamidino-2-phenylindole staining). Values are means ± SEM of 50 different fields (0.1 mm2) from four different animals of each genotype. Data were analyzed by Student's t test.
Hormone Analysis.
Blood samples were centrifuged at 4,000 rpm for 15 min. Sera were removed and stored at −20°C. PRL was measured by a mouse PRL RIA kit, obtained from A. F. Parlow (Pituitary Hormones and Antisera Center, University of California Los Angeles Medical Center).
Cell Culture and Thymidine Labeling.
MMQ rat pituitary tumor cells were maintained under 5% CO2 in RPMI 1640 medium containing 10% horse serum and 5% FBS. MMQ cells were kept 24 h in 0.2% FCS before bromocriptine (500 nM) or phorbol 12-myristate 13-acetate (PMA) (100 nM) treatment. [3H]Thymidine was added for 3 h at 16 h after bromocriptine or PMA induction.
All of the inhibitors (U0126 at 10 μM, PD98059 at 50 μM, SB203580 at 5 μM, Ro318220 at 1 μM, and haloperidol 10 μM) were added 1 h before bromocriptine or PMA treatment. U0126, PD98059, SB203580, and Ro318220 were from Calbiochem. Bromocriptine, PMA, and haloperidol were from Sigma.
Immunoblotting Analysis.
Pituitary anterior lobes were rapidly dissected, frozen, and homogenized directly in 50 μl of Laemmli buffer (22). Protein extracts were resolved by standard SDS/PAGE and quantified by Coomassie blue staining. Samples were electroblotted onto Protan nitrocellulose (Schleicher & Schuell). Membranes were incubated in PBS/5% low-fat milk and the appropriate antibody for 16 h at 4°C. Donkey, anti-rabbit-horseradish peroxidase (Jackson) antibodies were used to reveal immunocomplexes by enhanced chemioluminescence (ECL; Amersham Pharmacia). After P-ERK incubation and Western blot analysis the membranes were stripped and incubated with ERK. ERK and P-ERK (New England Biolabs) were used at 1:1,000. Protein bands were quantified with a Bio-Rad GS-700 Imaging Densitometer. Each P-ERK value was normalized to total ERK level. The time of exposure of the Western blots chosen to illustrate the level of P-ERK and ERK are different in all of the figures.
Results
Mouse Transgenic Lines Overexpressing D2L or D2S Receptors.
The physiological relevance of the two different isoforms on dopamine (DA)-mediated signaling is still unknown. Modifications of the D2L/D2S ratio might affect D2R-controlled functions. The pituitary gland, and in particular the lactotrops, represent an interesting model to address this question because evaluation of PRL levels, both at the mRNA and protein levels, represents an easy readout of D2R-mediated activities.
Two transgenic constructs were generated in which mouse D2L or D2S cDNAs were placed under the control of the 3-kb upstream region of the rat prolactin promoter (18, 23) (Fig. 1A). The polyadenylation (pA) site of the SV40 virus (19) was added at the 3′ extremity of the D2R cDNAs, which allowed the transgenes to be recognized easily from the endogenous D2R gene in vivo. Among several lines obtained and characterized, we chose two lines having integrated an equal number of transgene copies (30 copies), indicated here as PRL-D2L and PRL-D2S, to perform our studies. The presence and integrity of the constructs in transgenic mice tail DNA were analyzed by Southern blot by using the SV40 pA site as probe (Fig. 1B). Northern blot analysis of RNA prepared from different tissues revealed specific expression of the transgene only in the pituitary of both PRL-D2S and PRL-D2L mice (Fig. 1C). In situ hybridization on transgenic mouse pituitaries showed a clear colocalization in lactotrops of D2R and PRL probes (data not shown), in agreement with the specific expression of PRL promoter only in lactotrops (18, 23).
Overexpression of D2L and D2S was confirmed, at the mRNA and protein levels by performing RNase protection (Fig. 1D) and ligand-binding assays (Fig. 1E). RNase protection analyses showed that, whereas in the WT the D2L:D2S ratio is 8:1, in PRL-D2L it becomes 24:1 and in PRL-D2S 1:20. Ligand-binding assays supported these data showing a 3-fold increase in the number of D2-binding sites (Bmax) in the pituitary of both transgenic mice lines as compared with WT littermates (Fig. 1E) and similar affinity (Kd) for spiperone in all groups (Fig. 1E). These results also indicate that although D2R-mediated signaling negatively modulates the PRL promoter, this regulation is not sufficient to counteract completely the expression of the transgenes.
Modified Pituitary Anatomy in Transgenic Mice.
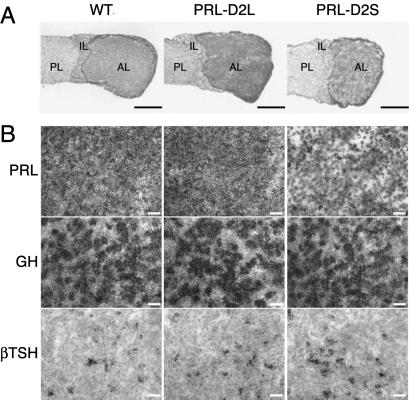
Next we analyzed the influence of the increase of either D2L or D2S sites on the pituitary of transgenic animals. Macroscopic observations of the pituitary glands from transgenic and WT female mice showed a clear reduction in size of the pituitary anterior lobe in PRL-D2S mice in comparison with mice of WT and PRL-D2L genotype (Fig. 2A), which was evidenced by a 15% reduction in the weight of PRL-D2S pituitaries (1.95 ± 0.1, n = 4) in comparison with those of WT and PRL-D2L mice (2.3 ± 0.15, n = 4). In situ hybridizations with a mouse PRL probe on pituitaries from 4-month-old WT, PRL-D2L, and PRL-D2S female mice were performed. PRL mRNAs both by in situ hybridization and Northern analysis were reduced by almost 80% in PRL-D2S pituitaries as compared with WT and PRL-D2L glands (Figs. 2B and 3C), in agreement with an inhibitory role of D2S on PRL synthesis. We also analyzed the presence of possible alterations of the thyrotrop and somatotrop cells. These two cell types belong to the same lineage and are precursors of the lactotrops (24–26). In situ hybridizations performed by using the β-thyroid-stimulating hormone and growth hormone probes showed no difference either in the number of thyrotrops and somatotrops or in the level of expression of the hormones they produced (Fig. 2B). Similarly, analyses of the gonadotrops and corticotrops of transgenic pituitaries by using specific probes did not reveal differences as compared with WT pituitaries (data not shown).
Fig 2.
Analyses of thyro-somato-mammotrop lineage in pituitaries from 4-month-old WT, PRL-D2L, and PRL-D2S mice. (A) Cryostat sections were postfixed in formalin and stained by toluidine blue. The size of anterior lobe of pituitaries from PRL-D2S mice is reduced as compared with WT and PRL-D2L glands. Quantification of the pituitary anterior lobe surface in PRL-D2S versus WT mice (n = 7) displayed a reduction of 30 ± 5% in size without cellular density modification. PL, posterior lobe; IL, Intermediate lobe; AL, anterior lobe. (Bars = 200 μM.) (B) Hormone expression and cell number were evaluated by in situ hybridizations on serial pituitary sections by using the mouse PRL, growth hormone (GH), and β thyroid stimulating hormone (TSH) antisense probes. Genotypes are as indicated. Sections were dipped in Kodak NTB-2 autoradiography emulsion and exposed for 24 h. Nuclei were counterstained with toluidine blue. A strong reduction of PRL mRNA-expressing cells was observed in PRL-D2S mice. (Bars = 20 μM.)
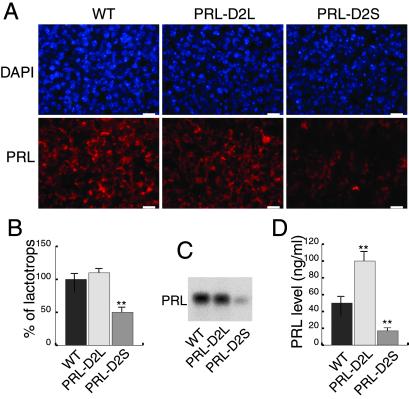
Fig 3.
Quantitative analyses of PRL expression at mRNA, protein, and serum levels in WT versus transgenic mice. (A) Lactotrops were identified by using a rabbit anti-PRL antibody. Nuclei were visualized by 4′,6-diamidino-2-phenylindole (DAPI) staining. Note the prominent decrease in PRL expressing cells in PRL-D2S mice. (Bars < 10 μM.) (B) The number of lactotrops was estimated by counting the number of PRL-positive cells (Cy3, red staining) over the total number of nuclei (DAPI staining). Values are means ± SEM of 50 different fields (0.1 mm2) from four different animals of each genotype as indicated. The WT value was arbitrarily taken as 100%. **, P < 0.01 (Student's t test). (C) Northern blot analyses of PRL mRNA expression in the pituitary of female animals from the three genotypes, as indicated. (D) Quantifications of PRL serum levels in WT, PRL-D2L, and PRL-D2S mice performed by RIA with a mouse PRL antibody. The values are expressed in nanograms of PRL per milliliter of serum. n = 7 per each genotype; **, P < 0.01 versus wild-type mice (Student's t test).
Parallel immunofluorescence analyses performed on these pituitaries by using an antibody directed against PRL showed a 50% decrease in the lactotrop number in the anterior lobe of PRL-D2S versus WT (Fig. 3 A and B). Conversely, a trend toward an increase of the lactotrop cell population was noticed in the PRL-D2L mice, although after quantification this increase never reached statistical significance. In agreement with in situ hybridization data, a strong decrease of PRL mRNA was observed by Northern blot analysis in PRL-D2S mice as compared with PRL-D2L and WT pituitaries (Fig. 3C). In addition to PRL synthesis, DA also controls PRL release (5). Thus, we measured PRL plasma levels in animals of the three genotypes by RIA (Fig. 3D). PRL plasma levels were reduced by 70% in PRL-D2S mice as compared with WT littermates, reflecting the reduction of lactotrops. Surprisingly, we found a significant 50% increase of PRL levels in PRL-D2L mice, which seems very likely dependent on an increase of PRL release.
These results suggest that overexpression of either D2L or D2S differently affects PRL synthesis and release. Furthermore, although D2S overexpression has a strong inhibitory effect both on PRL mRNA expression and lactotrop proliferation, D2L only affects PRL release.
Lactotrop Proliferation and ERKs Activation.
Ligand activation of the D2R elicits a rapid phosphorylation of ERKs both in vitro (27–30) and in vivo (31). Furthermore, ERK activation has been correlated with growth arrest or proliferation depending of the cellular type (32).
MMQ cells, a lactotrop cell line endogenously expressing functional DA D2 receptors (Fig. 4A) (33, 34), were used to investigate whether the MAPK pathway might modulate lactotrop proliferation. Cells were treated with bromocriptine, a specific D2R agonist at the concentration of 500 nM (35).
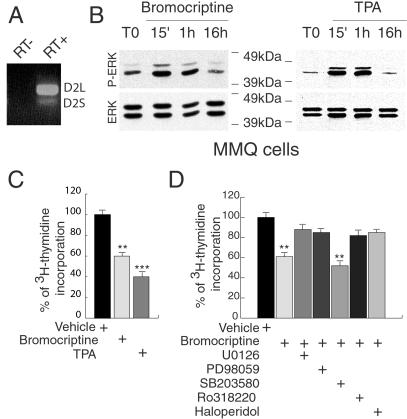
Fig 4.
D2 receptor-mediated effects on ERKs phosphorylation and cellular proliferation in the MMQ lactotrop cell line. (A) RT-PCR analysis of the expression of the endogenous D2R isoforms in MMQ cells. (B) Western blots of the time course of ERK activation in MMQ after addition in the medium of 500 nM bromocriptine or 100 nM PMA (TPA). Total level of ERK proteins in each sample is shown as internal control of loaded quantities of protein extracts. (C) Inhibitory effect of bromocriptine and PMA on MMQ cell proliferation measured by [3H]thymidine incorporation. The vehicle value was arbitrarily taken as 100%. **, P < 0.01; ***, P < 0.001 versus vehicle (Student's t test). (D) Effect of different inhibitors and D2R antagonist (U0126 at 10 μM, PD98059 at 50 μM, SB203580 at 5 μM, Ro318220 at 1 μM, haloperidol at 10 μM) on [3H]thymidine incorporation after bromocriptine treatment of MMQ cells. The value of [3H]thymidine incorporation only in the presence of the inhibitors was arbitrarily taken as 100%. **, P < 0.01 versus vehicle (Student's t test).
Activation of the MAPK pathway was assessed by Western blot analyses by using specific phospho-ERKs antibodies. An increase of ERK phosphorylation is observed 15 min after bromocriptine was added to the medium (Fig. 4B). The activation is still sustained 1 h after the addition of the compound and disappears after 16 h. To analyze whether activation of the MAPK pathway has consequences on lactotrop cell proliferation, cells were incubated with [3H]thymidine 16 h after bromocriptine addition, harvested after three additional hours, and the radioactivity measured. A significant reduction (40%) in the incorporation of tritiated thymidine was observed in bromocriptine-treated MMQ cells (Fig. 4C). This effect was completely blocked by haloperidol, a D2R-specific antagonist (Fig. 4D). To establish the link between ERKs activation and lactotrop proliferation, we used PMA, a well established stimulator of ERKs phosphorylation (36, 37). Addition of 100 nM PMA to the cells resulted into a strong activation of ERKs phosphorylation (Fig. 4B). The kinetics of ERK phosphorylation elicited by PMA and bromocriptine followed a similar time course. PMA also induced a 52% reduction of thymidine incorporation in MMQ cells (Fig. 4C). bromocriptine effect on cell proliferation is blocked by pretreatment with U0126 or PD98059, two specific inhibitors of ERKs activation, whereas no effect was observed by using the SB203580, a specific inhibitor of the p38 kinase (Fig. 4D). These data clearly indicate that in lactotrops the activation of the ERK pathway by D2R stimulation is involved in the control of the proliferation rate of these cells. Ro318220, a selective inhibitor of protein kinase C (PKC), is also able to revert bromocriptine effect (Fig. 4D) suggesting a possible role of PKC activation in D2R signaling. Similarly, the PMA-induced proliferation was partially blocked by pretreatment with U0126, PD98059, and Ro318220 (data not shown). These data indicate that stimulation of the MAPK pathway by D2R leads to a significant reduction of proliferation in a lactotrop cell line.
Is ERKs Phosphorylation D2R Isoform-Dependent in Vivo?
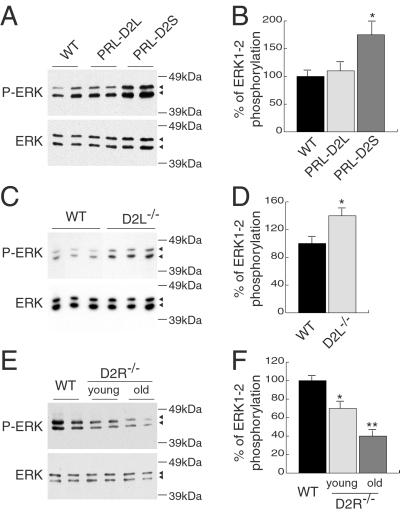
Western blot analyses with pituitary anterior lobe extracts from transgenic and WT female mice showed a clear increase of ERKs phosphorylation (P-ERK) specifically in PRL-D2S (Fig. 5A), but not in PRL-D2L animals. Quantification of these results showed a 75% increase of P-ERKs in PRL-D2S compared with WT littermates and PRL-D2L mice (n = 8) (Fig. 5B). Induction of ERKs phosphorylation in PRL-D2S mice (Fig. 5 A and B) and the observed reduction in lactotrops number (Fig. 3 A and B) correlate well with our in vitro data (Fig. 4). In particular, activation of ERKs seems to be strictly correlated with the overexpression of the D2S isoform. The role of D2S in the control of MAPK activation was further analyzed measuring the P-ERK level in the anterior lobe of D2L−/− mice (15). In these mice the total number of D2R sites are formed by D2S. In agreement with the results obtained in PRL-D2S transgenic mice, we found a 40% increase of ERK in the anterior lobes of D2L−/− mice as compared with WT littermates (Fig. 5 C and D).
Fig 5.
Analysis of P-ERK levels in the anterior pituitaries of WT, PRL-D2L, PRL-D2, D2L−/−, and D2R−/− female mice. (A) Analysis of protein extracts from pituitary anterior lobes of WT and PRL-D2L, PRL-D2S female transgenic mice by using an anti-P-ERK antibody. To control for variation in the loaded amount of proteins, the same filter was then stripped and incubated with an antibody anti-ERK. Note that the time of exposure to visualize P-ERK and ERK proteins is different. (B) Quantification of P-ERK level in WT, PRL-D2L, and PRL-D2S female mice pituitaries. Each P-ERK level was normalized to the total ERK level. n = 8; *, P < 0.05 versus wild-type mice (Student's t test). (C) P-ERK and ERK protein levels in WT and D2L−/− pituitary anterior lobes. (D) Quantification of P-ERKs level in WT and D2L−/− female mice pituitaries. n = 7; *, P < 0.05 versus wild-type mice (Student's t test). (E) P-ERK and ERK protein levels in pituitary anterior lobe of WT and D2R−/− female mice, 2 or 8 months old, as indicated. For the WT, only the extracts corresponding to old animals are shown because no difference in ERK proteins exists either in the total or the phosphorylated levels between the two age groups. (F) Quantification of P-ERK level in WT, young and old D2R−/− female mice pituitaries. n = 7; *, P < 0.05; **, P < 0.01 versus wild-type mice (Student's t test).
Thus, these data show that in vivo, changing the physiological D2L/D2S ratio in favor of D2S leads to an increased activation of the MAPK pathway by DA under basal conditions.
Reduced Levels of P-ERK and Abnormal Pituitary Proliferation in D2R−/− Mice.
The hypoplasia observed on overexpression of the D2S isoform in PRL-D2S mice prompted us to revisit the pituitary tumor phenotype observed in the D2R-null mice (2) to evaluate the state of ERK phosphorylation in lactotrops lacking all forms of D2R.
Western blot analyses of pituitary extracts prepared from anterior lobes of 8-month-old D2R−/− and WT female mice were performed. A 60% significant decrease in the levels of P-ERKs was found in the anterior lobe of D2R−/− as compared with WT female littermates (Fig. 5 E and F). To test whether the reduced phosphorylation of ERKs is a secondary event in tumor formation or a primary consequence of the absence of D2R, we also analyzed P-ERKs levels in young females, which do not exhibit pituitary hyperplasia yet (2). Protein extracts were prepared from the anterior lobes of 2-month-old females of both genotypes. Western blot analysis revealed a 30% reduction of ERKs phosphorylation in young female D2R−/− mice as compared with their WT siblings (Fig. 5 E and F). The lower reduction of ERKs phosphorylation in young compared with older D2R−/− females thus correlates with the advancing state of the hyperplasia.
Discussion
DA is a key regulator of lactotrop functions. The analysis of mutants lacking of D2R (2, 6) or the DA transporter (38), elucidated an additional function related to the control of lactotrop cell proliferation. Indeed, absence of control by DA as in D2R−/− mice or high level of DA as in DA transporter-null mice, lead to the development of tumors of lactotrop origin (prolactinomas) or to pituitary hypoplasia, respectively. The mechanisms underlying this control are of both a biological and a therapeutical interest because prolactinomas are frequent tumors in humans. In addition to the inhibition of the adenylyl cyclase, D2R has been reported to regulate other transduction pathways also (3) leading to the elevation of intracellular calcium as well as in the activation of PKC and MAPK pathways (3, 27). The presence of two isoforms of D2R, expressed in a ratio that favors D2L with respect to D2S in the pituitary, questions its physiological significance. To address this issue we have altered this ratio by generating transgenic mice overexpressing either D2L or D2S in the lactotrops.
Overexpression of D2L and D2S Differently Affects Lactotrop Number and PRL Levels.
Our data show that overexpression of either D2L or D2S differently affects lactotrop physiology in vivo. This overexpression has a major impact on the regulation of PRL secretion. Indeed, the strong reduction of PRL observed in PRL-D2S mice indicates that D2S is very likely the isoform regulating PRL synthesis and lactotrop cell number. On the other hand D2L activation plays a major role in the regulation of PRL release. Therefore, we propose that in WT mice a strict control over the D2L/D2S ratio is required to maintain a normal lactotrop number and PRL synthesis, very likely due to D2S, and release mainly controlled by D2L. Estrogen treatment of normal mice, which leads to an increase of PRL levels, has also been reported to increase the ratio of the D2 isoforms toward D2S. It might be speculated that the estrogen-induced increase of D2S might help negatively regulate PRL synthesis and lactotrop proliferation (39).
D2R-Dependent ERKs Phosphorylation in Lactotrops.
The implication of MAPK pathway in the signaling mediated by D2R has recently been proposed both in vitro (27–29) and in vivo (31). Our experiments on MMQ cells show that bromocriptine stimulation of D2R results in a prolonged activation of ERKs and in a concomitant reduction of the proliferation rate of these cells. These effects are blocked by haloperidol and by specific MEK1/2 inhibitors, but not by a specific p38 kinase inhibitor. ERK phosphorylation induced by the stimulation of D2R in MMQ cells is mediated by PKC activation as it is in the brain (27), because it is abolished by a specific PKC inhibitor. The time course of MAPK phosphorylation seems to be a direct indicator of cell cycle progression. Prolonged kinetics of activation of this pathway have been related to effects on cell differentiation, whereas rapid kinetics have been linked to proliferation (40). Our results on MMQ cells strengthen these reports and support the notion that a sustained activation of ERKs phosphorylation is accompanied by a reduction of the proliferation rate. It is thus tempting to speculate that D2R activation of the MAPK pathway in vivo follows the same mechanism.
ERK Activation in the Control of Lactotrop Cell Proliferation.
Overexpression of D2S leads to the development of hypoplastic pituitaries in which the level of phosphorylated ERKs is significantly increased as compared with WT or PRL-D2L mice. This finding is also supported by the observation of a higher basal level of P-ERK in the anterior lobe of D2L−/− mice (15), where all of the D2R sites are composed by D2S.
The tight correlation between ERKs phosphorylation and lactotrop proliferation is further supported by the analysis of D2R−/− mice, where the basal level of ERKs phoshorylation is significantly decreased as compared with their WT littermates. This phenomenon is dependent on the absence of D2R-mediated signaling and is not a consequence of the hyperplasia present in D2R-null mice. Indeed, the reduction of ERKs phosphorylation in D2R−/− mice is present also in young D2R−/− females at stages in which the hyperplasia is not yet developed. The lower level of P-ERKs in old versus young females might be explained either by the higher number of lactotrops present in the tumor or by the selection of a lactotrop population having some proliferative advantages. Interestingly, overexpression of D2L in PRL-D2L mice does not lead to MAPK activation.
We have previously shown that D2L and D2S have distinct functions in vivo in the CNS (15, 16), which seems to be the case also in the pituitary. Indeed, overexpression of the D2 isoforms differently affects the MAPK pathway, lactotrop cell proliferation, and PRL levels.
It might be speculated that through the interaction with different G proteins (41, 42), D2L might mainly exert its effect through a direct coupling with ion channels facilitating hormone release, whereas D2S would control PRL levels, principally at the mRNA level, through a preferential interaction with the cAMP pathway. The increased PRL release or the stronger activation of the ERK pathway observed in presence of overexpressed D2L or D2S receptors in transgenic mice suggests that altering the natural balance between D2S and D2L pushes toward a specific signaling route. In these conditions D2 isoforms might acquire specificity or better affinities for particular G proteins or effectors.
Overexpression of a single D2 isoform in the absence of the other, either in vitro (27, 40) or in vivo, might alter the signaling normally mediated by the presence of the two isoforms. This finding is also inferred by our preliminary results, because mating of D2R−/− with PRL-D2L or PRL-D2S transgenic mice leads in both cases to lactotrop hypoplasia and a concomitant increase of P-ERKs (T.A.S., C.I., and E.B., unpublished observations). In these mice, however. the overexpression of each isoform in lactotrops is very likely paralleled by high DA level. Indeed, lack of D2R in knockout mice results in the loss of autoreceptor functions regulating DA release (43). It is thus conceivable that a higher level of DA released in the portal vein system of D2R−/− mice upon physiological stimulation of hypothalamic DA neurons together with isoform overexpression in D2R−/− PRL-D2L or D2S mice leads to the described phenotype. The dependence of D2R-mediated signaling on a fixed D2L/D2S ratio strongly suggest an interaction between the two isoforms. Many aspects of D2 receptor physiology are still to unravel. Whether interference exist between D2L and D2S mRNAs for their translation, in the mechanisms regulating the trafficking of these isoforms at the membrane and whether D2L and D2S might heterodimerize in vivo is still not known. However, it seems that both receptors, in an adequate ratio, are required for the full expression of D2 normal functions. Future studies shall be aimed at identifying the functional relationship between the two D2 receptor isoforms, as well as their signaling cross-talk.
Acknowledgments
We acknowledge Dr. Marianne Le Meur for transgenic mice generation. We also acknowledge Dr. P. Sassone-Corsi, and all of the members of the laboratory for discussions. We thank Dr. Parlow and the National Hormone and Pituitary Program for pituitary antibodies, V. Giroult and E. Erbs for technical assistance, and J. L. Vonesch for image analysis. C.I. was supported by European Economic Community (CEE) and Fondation pour la Recherche Médicale (FRM), La Ligue contre le Cancer fellowships. This work was supported by funds from Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Centre Hospitalo-Universitaire de Strasbourg, and Association pour la Recherche sur le Cancer (to E.B.).
Abbreviations
PRL, prolactin
D2R, dopamine D2 receptor
ERK, extracellular-regulated kinase
MAPK, mitogen-activated protein kinase
DA, dopamine
SV40, simian virus 40
PMA, phorbol 12-myristate 13-acetate
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Baik J. H., Picetti, R., Saiardi, A., Thiriet, G., Dierich, A., Depaulis, A., Le Meur, M. & Borrelli, E. (1995) Nature 377, 424-428. [DOI] [PubMed] [Google Scholar]
- 2.Saiardi A., Bozzi, Y., Baik, J. H. & Borrelli, E. (1997) Neuron 19, 115-126. [DOI] [PubMed] [Google Scholar]
- 3.Picetti R., Saiardi, A., Abdel Samad, T., Bozzi, Y., Baik, J. H. & Borrelli, E. (1997) Crit. Rev. Neurobiol. 11, 121-142. [DOI] [PubMed] [Google Scholar]
- 4.Elsholtz H. P., Lew, A. M., Albert, P. R. & Sundmark, V. C. (1991) J. Biol. Chem. 266, 22919-22925. [PubMed] [Google Scholar]
- 5.Ben-Jonathan N. (1985) Endocr. Rev. 6, 564-589. [DOI] [PubMed] [Google Scholar]
- 6.Kelly M. A., Rubinstein, M., Asa, S. L., Zhang, G., Saez, C., Bunzow, J. R., Allen, R. G., Hnasko, R., Ben-Jonathan, N., Grandy, D. K., et al. (1997) Neuron 19, 103-113. [DOI] [PubMed] [Google Scholar]
- 7.Asa S. L., Kelly, M. A., Grandy, D. K. & Low, M. J. (1999) Endocrinology 140, 5348-5355. [DOI] [PubMed] [Google Scholar]
- 8.Wass J. A., Besser, G. M. & McDonald, W. I. (1982) Br. Med. J. (Clin. Res. Ed.) 285, 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molitch M. E., Elton, R. L., Blackwell, R. E., Caldwell, B., Chang, R. J., Jaffe, R., Joplin, G., Robbins, R. J., Tyson, J. & Thorner, M. O. (1985) J. Clin. Endocrinol. Metab. 60, 698-705. [DOI] [PubMed] [Google Scholar]
- 10.Dal Toso R., Sommer, B., Ewert, M., Herb, A., Pritchett, D. B., Bach, A., Shivers, B. D. & Seeburg, P. H. (1989) EMBO J. 8, 4025-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monsma F. J., McVittie, L. D., Gerfen, C. R., Mahan, L. C. & Sibley, D. R. (1989) Nature 342, 926-929. [DOI] [PubMed] [Google Scholar]
- 12.Giros B., Sokoloff, P., Martres, M. P., Riou, J. F., Emorine, L. J. & Schwartz, J. C. (1989) Nature 342, 923-926. [DOI] [PubMed] [Google Scholar]
- 13.Montmayeur J. P., Bausero, P., Amlaiky, N., Maroteaux, L., Hen, R. & Borrelli, E. (1991) FEBS Lett. 278, 239-243. [DOI] [PubMed] [Google Scholar]
- 14.Guiramand J., Montmayeur, J. P., Ceraline, J., Bhatia, M. & Borrelli, E. (1995) J. Biol. Chem. 270, 7354-7358. [DOI] [PubMed] [Google Scholar]
- 15.Usiello A., Baik, J. H., Rouge-Pont, F., Picetti, R., Dierich, A., LeMeur, M., Piazza, P. V. & Borrelli, E. (2000) Nature 408, 199-203. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Xu, R., Sasaoka, T., Tonegawa, S., Kung, M. P. & Sankoorikal, E. B. (2000) J. Neurosci. 20, 8305-8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan Z. U., Mrzljak, L., Gutierrez, A., de la Calle, A. & Goldman-Rakic, P. S. (1998) Proc. Natl. Acad. Sci. USA 95, 7731-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borrelli E., Sawchenko, P. E. & Evans, R. M. (1992) Proc. Natl. Acad. Sci. USA 89, 2764-8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramani S. & Southern, P. J. (1983) Anal. Biochem. 135, 1-15. [DOI] [PubMed] [Google Scholar]
- 20.Hogan B., Costantini, F. & Lacy, E., (1986) Manipulating the Mouse Embryo: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 21.Guivarc'h D., Vincent, J. D. & Vernier, P. (1998) Endocrinology 139, 4213-4221. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U. K. (1970) Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 23.McAndrew J., Paterson, A. J., Asa, S. L., McCarthy, K. J. & Kudlow, J. E. (1995) Endocrinology 136, 4479-4488. [DOI] [PubMed] [Google Scholar]
- 24.Behringer R. R., Mathews, L. S., Palmiter, R. D. & Brinster, R. L. (1988) Genes Dev. 2, 453-461. [DOI] [PubMed] [Google Scholar]
- 25.Voss J. W. & Rosenfeld, M. G. (1992) Cell 70, 527-530. [DOI] [PubMed] [Google Scholar]
- 26.Borrelli E. (1994) Trends Genet. 10, 222-224. [DOI] [PubMed] [Google Scholar]
- 27.Yan Z., Feng, J., Fienberg, A. A. & Greengard, P. (1999) Proc. Natl. Acad. Sci. USA 96, 11607-11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh G. I., Hall, D. A., Warnes, A., Strange, P. G. & Proud, C. G. (1998) J. Neurochem. 70, 2139-2146. [DOI] [PubMed] [Google Scholar]
- 29.Oak J. N., Lavine, N. & Van Tol, H. H. (2001) Mol. Pharmacol. 60, 92-103. [DOI] [PubMed] [Google Scholar]
- 30.Vindis C., Seguelas, M. H., Lanier, S., Parini, A. & Cambon, C. (2001) Kidney Int. 59, 76-86. [DOI] [PubMed] [Google Scholar]
- 31.Cai G., Zhen, X., Uryu, K. & Friedman, E. (2000) J. Neurosci. 20, 1849-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis T. S., Shapiro, P. S. & Ahn, N. G. (1998) Adv. Cancer Res. 74, 49-139. [DOI] [PubMed] [Google Scholar]
- 33.Judd A. M., Login, I. S., Kovacs, K., Ross, P. C., Spangelo, B. L., Jarvis, W. D. & MacLeod, R. M. (1988) Endocrinology 123, 2341-2350. [DOI] [PubMed] [Google Scholar]
- 34.Steffey M. E., Roberts, E., Frail, D. E., Kebabian, J. W. & MacKenzie, R. G. (1993) Biochem. Pharmacol. 46, 747-751. [DOI] [PubMed] [Google Scholar]
- 35.Arita J., Hashi, A., Hoshi, K., Mazawa, S. & Suzuki, S. (1998) Neuroendocrinology 68, 163-171. [DOI] [PubMed] [Google Scholar]
- 36.Lange-Carter C. A. & Johnson, G. L. (1994) Science 265, 1458-1461. [DOI] [PubMed] [Google Scholar]
- 37.Alblas J., Slager-Davidov, R., Steenbergh, P. H., Sussenbach, J. S. & van der Burg, B. (1998) Oncogene 16, 131-139. [DOI] [PubMed] [Google Scholar]
- 38.Bosse R., Fumagalli, F., Jaber, M., Giros, B., Gainetdinov, R. R., Wetsel, W. C., Missale, C. & Caron, M. G. (1997) Neuron 19, 127-138. [DOI] [PubMed] [Google Scholar]
- 39.Guivarc'h D., Vernier, P. & Vincent, J. D. (1995) Neuroscience 69, 159-166. [DOI] [PubMed] [Google Scholar]
- 40.Qui M. S. & Green, S. H. (1992) Neuron 9, 705-717. [DOI] [PubMed] [Google Scholar]
- 41.Jackson D. M. & Westlind-Danielsson, A. (1994) Pharmacol. Ther. 64, 291-370. [DOI] [PubMed] [Google Scholar]
- 42.Missale C., Nash, S. R., Robinson, S. W., Jaber, M. & Caron, M. G. (1998) Physiol. Rev. 78, 189-225. [DOI] [PubMed] [Google Scholar]
- 43.Rouge-Pont F., Usiello, A., Benoit-Marand, M., Gonon, F., Piazza, P. V. & Borrelli, E. (2002) J. Neurosci. 22, 3293-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]