Abstract
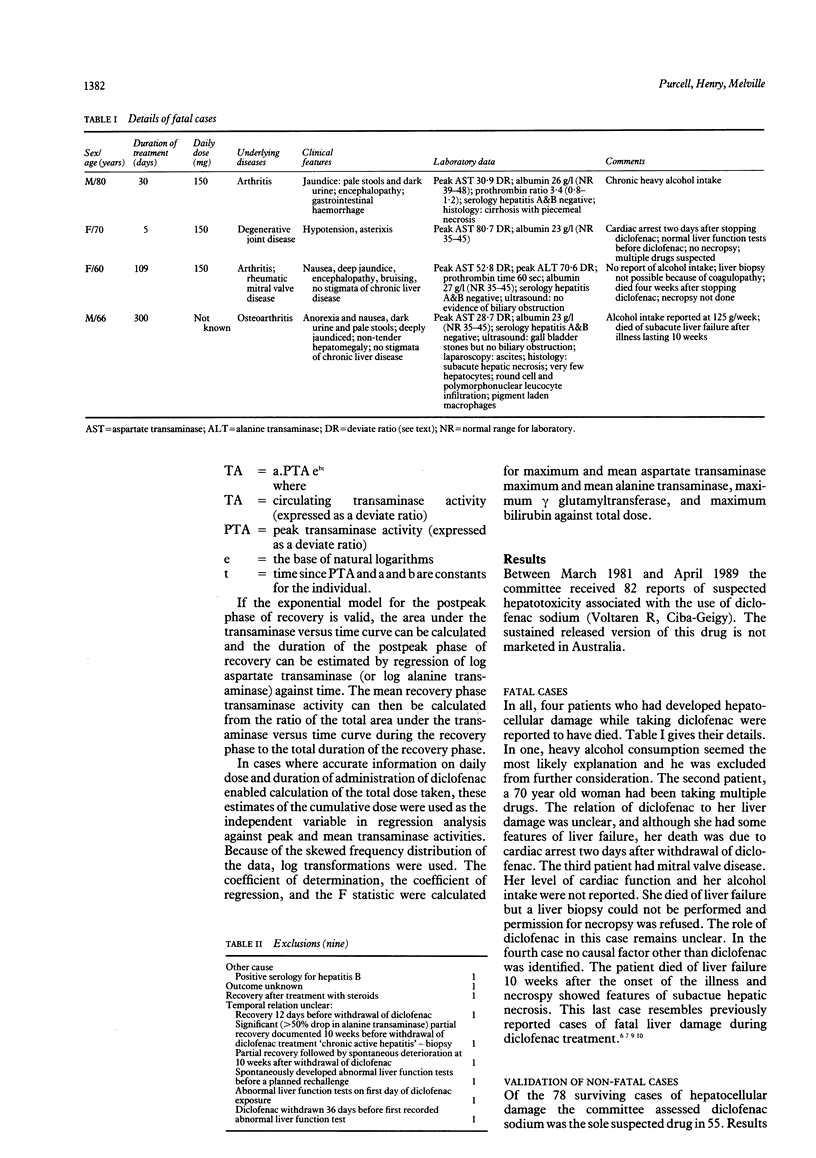
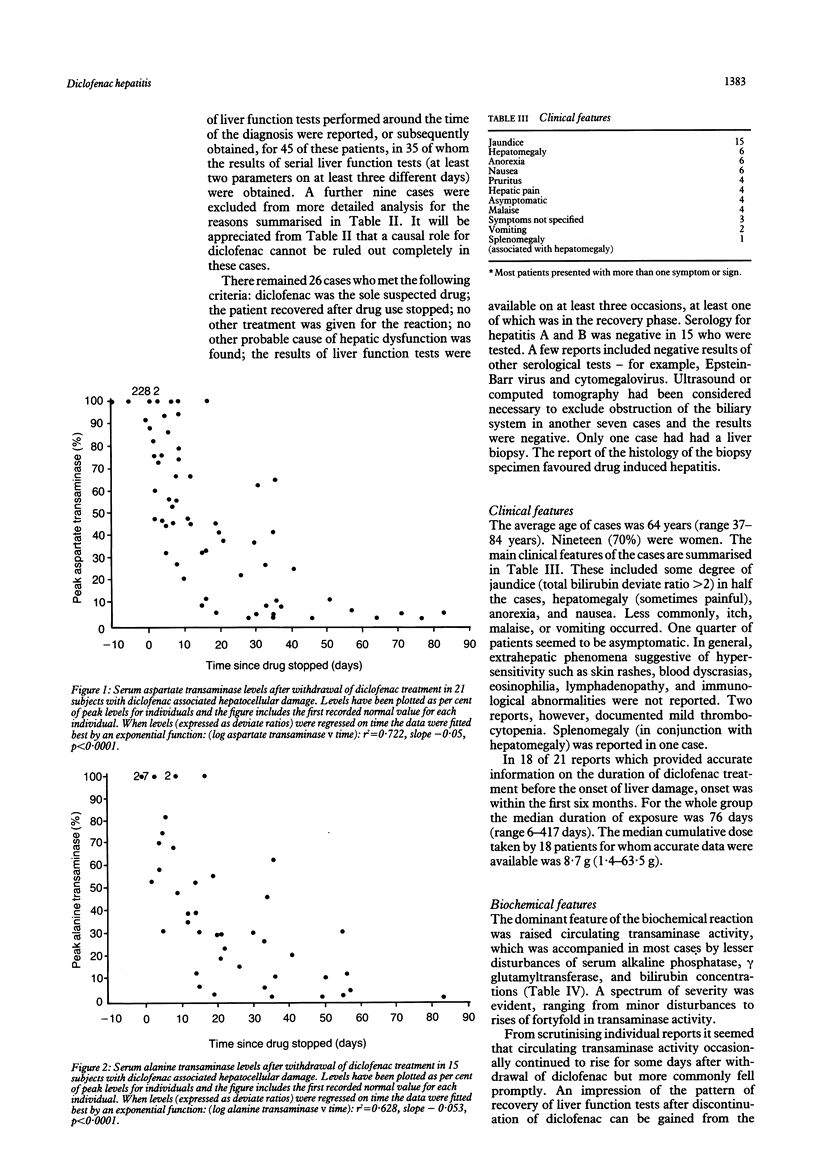
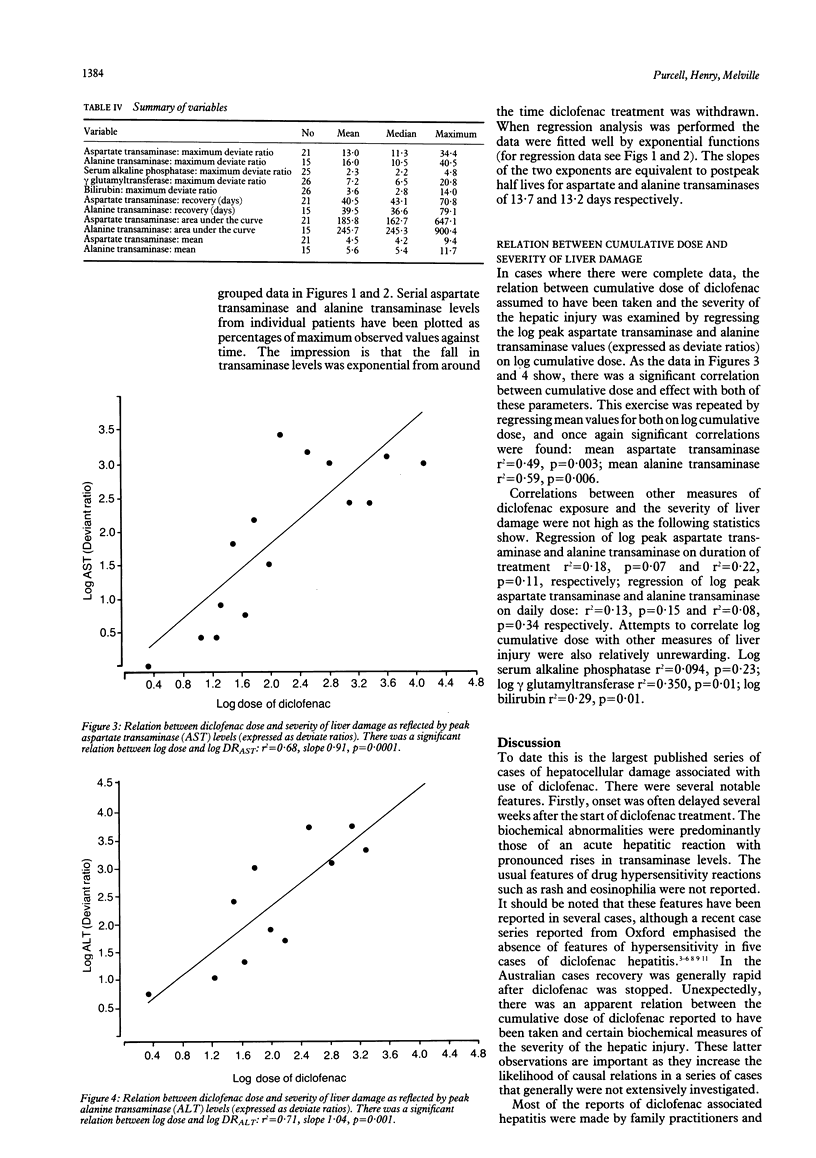
The characteristics of liver damage associated with the use of diclofenac, a popular nonsteroidal anti-inflammatory drug, were investigated by reviewing adverse drug reaction reports for Australia. Twenty six patients were reported for whom diclofenac was the sole suspected drug cause of their liver damage. The average age of the patients was 64 years (range 37-84 years); 19 (70%) were women. The most common clinical features were jaundice, hepatomegaly, anorexia, and nausea. Features of drug hypersensitivity were not reported. Duration of treatment with diclofenac before the onset of the illness ranged from 6-417 days (median 76 days). The most prominent biochemical abnormalities were raised serum aspartate transaminase and alanine transaminase activity of up to 30 to 40 times the upper limit of the normal range. Recovery generally started soon after withdrawal of diclofenac and the decrease in aspartate transaminase and alanine transaminase for the group was exponential, with half lives of around 13 days. The average total dose taken by 18 patients for whom accurate data were available was 8.7 g (range 1.4-63.5 g) and, unexpectedly, there was a significant relation between the logarithm of the dose of diclofenac and the logarithms of the peak and mean transaminase levels. Hepatocellular damage during treatment with diclofenac seems to be a rare event. From this analysis of Australian reports it seems that in a small subgroup of patients liver injury may be a direct toxic effect of diclofenac or a metabolite.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babany G., Bernuau J., Danan G., Rueff B., Benhamou J. P. Hépatite fulminante chez une femme prenant de la glafénine et du diclofénac. Gastroenterol Clin Biol. 1985 Feb;9(2):185–185. [PubMed] [Google Scholar]
- Babany G., Pessayre D., Benhamou J. P. Hépatite au diclofénac. Gastroenterol Clin Biol. 1983 Mar;7(3):316–316. [PubMed] [Google Scholar]
- Breen E. G., McNicholl J., Cosgrove E., McCabe J., Stevens F. M. Fatal hepatitis associated with diclofenac. Gut. 1986 Nov;27(11):1390–1393. doi: 10.1136/gut.27.11.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes P., Leloet X., Bercoff E., Fouin-Fortunet H. Hepatite au diclofénac. Une observation. Presse Med. 1984 Sep 1;13(30):1847–1847. [PubMed] [Google Scholar]
- Dunk A. A., Walt R. P., Jenkins W. J., Sherlock S. S. Diclofenac hepatitis. Br Med J (Clin Res Ed) 1982 May 29;284(6329):1605–1606. doi: 10.1136/bmj.284.6329.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iveson T. J., Ryley N. G., Kelly P. M., Trowell J. M., McGee J. O., Chapman R. W. Diclofenac associated hepatitis. J Hepatol. 1990 Jan;10(1):85–89. doi: 10.1016/0168-8278(90)90077-5. [DOI] [PubMed] [Google Scholar]
- Lascar G., Grippon P., Lévy V. G. Hépatite aiguë mortelle au cours d'un traitement par le diclofénac (Voltarène). Gastroenterol Clin Biol. 1984 Nov;8(11):881–882. [PubMed] [Google Scholar]
- Paulus H. E. FDA Arthritis Advisory Committee meeting. Arthritis Rheum. 1982 Sep;25(9):1124–1125. doi: 10.1002/art.1780250914. [DOI] [PubMed] [Google Scholar]
- Schapira D., Bassan L., Nahir A. M., Scharf Y. Diclofenac-induced hepatotoxicity. Postgrad Med J. 1986 Jan;62(723):63–65. doi: 10.1136/pgmj.62.723.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder R. J., Dinant H. J., Stricker B. H. Dodelijke leverbeschadiging tijdens gebruik van diclofenac. Ned Tijdschr Geneeskd. 1987 Nov 14;131(46):2088–2090. [PubMed] [Google Scholar]


