Abstract
The Rab3 GDP/GTP exchange protein (Rab3 GEP) activates the Rab3 small GTP-binding protein (G protein) family, including Rab3A that is an important member controlling synaptic vesicle trafficking. Here, we examined the role of Rab3 GEP in regulating neurotransmitter release in autapses of mouse hippocampal neurons in culture. The release probability was markedly reduced in Rab3 GEP−/− neurons, whereas the readily releasable pool size was not different between WT and Rab3 GEP−/− neurons, indicating that Rab3 GEP up-regulates a postdocking step of synaptic exocytosis. Because Rab3A reportedly down-regulates Ca2+-triggered fusion of synaptic vesicles, these results provide evidence for a role of Rab3 GEP in the postdocking process distinct from Rab3A activation.
Rab3A, a member of the Rab small G protein family, is involved in the process of Ca2+-dependent neurotransmitter release (1–5). Rab3A activity is regulated by a GDP/GTP exchange protein (Rab3 GEP) (6), a Rab GDP dissociation inhibitor (Rab GDI), and a GTPase-activating protein (Rab3 GAP) (7, 8). The Rab3A recycling is coupled with synaptic vesicle trafficking as follows (1, 2): (i) GDP-Rab3A forms an inactive complex with Rab GDI and stays in the cytosol of nerve terminals. (ii) GDP-Rab3A released from Rab GDI is converted to GTP-Rab3A by Rab3 GEP. (iii) GTP-Rab3A binds effector molecules, Rabphilin-3 (9) and Rim (10), localized at synaptic vesicles and the active zone, respectively. These complexes facilitate translocation and docking of the synaptic vesicles to the active zone. (iv) GTP-Rab3A is converted to GDP-Rab3A by Rab3 GAP when the vesicles fuse with the presynaptic membrane. (v) GDP-Rab3A associated with Rab GDI is retrieved from the membrane to the cytosol. In Rab3A−/− mice, synaptic depression is increased during repetitive stimulation in the CA1 region of the hippocampus (11), and mossy fiber long-term potentiation (LTP) in the CA3 region is abolished (12). In Rab3A−/− hippocampal neurons in culture, the quantal release per synapse is increased, whereas the size of the readily releasable pool (RRP) measured by the application of hypertonic solution is normal (13). Thus, Rab3A appears to up-regulate the steps of translocation and docking, as well as to down-regulate the step of fusion.
We previously reported (14) knockout studies on Rab3A regulators, Rab GDIα and Rab3 GEP. Rab GDIα plays a specialized role in the Rab3A recycling to suppress hyperexcitability via modulation of presynaptic forms of plasticity. In Rab GDIα−/− mice, synaptic currents in the CA1 region of the hippocampus display larger enhancement during repetitive stimulation, which is apparently opposite to the phenotypes of Rab3A−/− mice. Epileptic seizures are readily induced in Rab GDIα−/− mice, which is consistent with observations in human X-linked mental retardation caused by mutations of the Rab GDIα gene (15). These results provide additional evidence that Rab3A up-regulates the steps of translocation and docking in neurotransmitter release. However, Rab3 GEP−/− mice appear to develop more profound impairment in neurotransmitter release than Rab3A−/− and Rab GDIα−/− mice (16). Rab3 GEP−/− mice die immediately after birth because of respiratory failure. The embryos at E18.5 show no evoked action potentials of the diaphragm and gastrocnemius muscles in response to electrical stimulation of the phrenic and sciatic nerves, respectively. The total number of the synaptic vesicles at the neuromuscular junction of Rab3 GEP−/− mice is remarkably reduced about one-tenth compared with WT mice. Thus, Rab3 GEP is essential for neurotransmitter release, at least in the peripheral nervous system, and it may be required for formation and trafficking of synaptic vesicles.
Because it is difficult to keep Rab3 GEP−/− mice alive up to 3 weeks when central synapses are formed and maturated, it remains unknown how Rab3 GEP functions in the central nervous system. Here, we explore the role of Rab3 GEP in neurotransmitter release by using autapses of hippocampal neurons cultured on glial microislands.
Materials and Methods
Culture.
WT and Rab3 GEP−/− mice have been described (16). The mice used for experiments were littermates from heterozygous interbreeding. Neuronal cells were cultured from the mouse hippocampus after collecting E18.5 embryos by Caesarian section from a mouse anaesthetized by diethyl ether. Culture methods were essentially the same as described (17, 18). Briefly, hippocampal regions were excised from mouse embryos, and cells were dispersed by trypsin treatment (0.25%, 15 min), and plated on glial microislands at a low density (2,000 cells per cm2). Neurons were cultured in DME medium (Sigma) supplemented with sera (10% FCS, 5% horse serum, and 1% rat serum) for the first 3 days, which were then replaced with serum-free, B27-supplemented (2%, GIBCO) DME medium containing dl-2-amino-5-phosphonovaleric acid (DL-AP5, 50 μM, Tocris, Bristol, U.K.) for 10–21 days under 5% CO2 at 37°C. Neurons solely grown on microislands were used for electrical recording.
Electrophysiology.
The autaptic evoked excitatory postsynaptic current (EPSC) was recorded using the whole-cell patch-clamp method (19) with a patch-clamp amplifier (EPC-7, List Electronics, Darmstadt, Germany), low-pass-filtered at 3 kHz, and digitally sampled at 10 kHz. The bath solution consisted of (in mM): NaCl 137, KCl 4, CaCl2 2, MgCl2 1, glucose 17, and Hepes-NaOH 10 (pH 7.4). Picrotoxin (50 μM) and DL-AP5 (50 μM) were also added during recording. The intrapipette solution consisted of (in mM): K-gluconate 129, KCl 30, MgCl2 2, EGTA 1, CaCl2 0.25, Na2ATP 3, GTP 0.3, and Hepes 5 (pH 7.2). Patch electrodes had resistances of 3–6 MΩ. Neurons were voltage clamped at −80 to −60 mV (usually −70 mV), and a 1-ms voltage step to +100 mV was applied, evoking an autaptic EPSC. A paired-pulse stimulus with an interval of 50 ms was applied every 20 s. Series resistance compensation was not used. Data were discarded when the change of series resistance was >30%. All experiments were performed at room temperature (25–27°C). A hypertonic solution (normal extracellular solution with 500 mM sucrose added) was applied to the autaptic neuron by using a puffer pipette controlled by Picospritzer (General Valve, Fairfield, NJ).
Most of the salts and chemicals were obtained from Wako Biochemicals (Osaka). EGTA and Hepes were from Dojindo laboratories (Kumamoto, Japan); K-gluconate, picrotoxin, and Cyclothiazide were from Sigma; and DL-AP5 was from Tocris.
Analysis.
Evoked EPSCs were stored on a DOS/V computer. All recording and analysis were carried out using pClamp8 (Axon). EPSC amplitude was measured after subtracting Na+-current, which was recorded from the response in NBQX (10 μM, Tocris) or from the failure response. Asynchronous mini-EPSCs were detected using the MINI ANALYSIS program (Jaejin Software, Leonia, NJ). Unless otherwise noted, results are reported as mean ± SEM, with the level of significance (P < 0.05) determined by the Student's t test.
Electron Microscopy.
Hippocampal neurons cultured on the glial microisland for 2 weeks were fixed either with a solution containing 2% glutaraldehyde and 2% paraformaldehyde or 2% paraformaldehyde in PBS (pH 7.4) for 4 h, for conventional electron microscopy or immunoelectron microscopy, respectively. These samples were processed ultrathin sections as described (20) and examined by an electron microscope (1010, JEOL, Tokyo).
Results
Down-Regulation of Excitatory Postsynaptic Current Amplitude in Rab3 GEP−/− Neurons.
We first investigated the role of Rab3 GEP in neurotransmitter release electrophysiologically by measuring the amplitudes of EPSCs in autapses of hippocampal neurons of WT and Rab3 GEP−/− mice. The amplitude of the evoked autaptic EPSC was smaller in Rab3 GEP−/− neurons (1,135 ± 192 pA, n = 34) than in WT neurons (5,111 ± 596 pA, n = 28) (Fig. 1 a and b, P < 0.001).
Fig 1.
Evoked autaptic EPSC in cultured hippocampal neurons. (a) Response to paired-pulse stimuli (50-ms interval) in WT neurons (a1) and Rab3 GEP−/− neurons (a2). (Bars, 2 nA, 50 ms.) (b) Amplitude of the first evoked EPSC in WT neurons (left column, n = 28) and Rab3 GEP−/− neurons (right column, n = 34). (c) The PPMR in WT neurons (left column, n = 28) and Rab3 GEP−/− neurons (right column, n = 34). ***, P < 0.001. (d) The rising time (10–90%) of the EPSC in WT neurons (left column, n = 28) and Rab3 GEP−/− neurons (right column, n = 34).
To estimate which side of the synapse, pre- or postsynapse, was affected in Rab3 GEP−/− neurons, responses to paired-pulse stimuli were analyzed. By applying paired pulses with 50-ms intervals to the cultured neurons, the paired-pulse modulation ratio (PPMR), defined as a relative amplitude of the second EPSC normalized by the first EPSC, was obtained and compared between WT and Rab3 GEP−/− neurons. The PPMR was 0.96 ± 0.02 (n = 28) in WT neurons, and 1.53 ± 0.08 (n = 34) in Rab3 GEP−/− neurons with a significant difference (Fig. 1c, P < 0.001). The rising time of the EPSC (time from 10 to 90%) was compared between WT and Rab3 GEP−/− neurons, but there was no difference in the rising time: 1.77 ± 0.17 ms (n = 14) in WT neurons and 1.53 ± 0.15 ms (n = 15) in Rab3 GEP−/− neurons, respectively (Fig. 1d). An enhanced PPMR in Rab3 GEP−/− neurons suggests that the presynaptic site is affected, even though other possibilities are not necessarily excluded by these methods.
Effects of High Ca2+ Concentration on the Autaptic EPSC.
Based on a hypothesis that the presynaptic release mechanism is affected in Rab3 GEP−/− neurons, effects of increase in Ca2+ concentration of the external solution ([Ca2+]out) on the synaptic release were compared between WT and Rab3 GEP−/− neurons. When [Ca2+]out was raised from 2 to 8 mM, the EPSC amplitude was significantly enhanced in both WT (Fig. 2 a1 and b, n = 8, P < 0.01) and Rab3 GEP−/− neurons (Fig. 2 a2 and b, n = 5, P < 0.05). There was no difference, however, in the enhanced ratio of the EPSC between WT and Rab3 GEP−/− neurons.
Fig 2.
Effects of high [Ca2+]out on autaptic EPSC. (a) Response to paired-pulse stimuli in WT neurons (a1) and Rab3 GEP−/− neurons (a2). Solid line, current trace record in 2 mM [Ca2+]out; dotted line, recorded in 8 mM [Ca2+]out. (Bars, 1 nA, 25 ms.) (b) The relative amplitude of the EPSC in 8 mM [Ca2+]out solution normalized by the EPSC amplitude recorded in 2 mM [Ca2+]out. Left column, WT neurons (n = 8); right column, Rab3 GEP−/− neurons (n = 5). (c) The PPMR in WT neurons (left column, n = 8) and Rab3 GEP−/− neurons (right column, n = 5).
Effects of [Ca2+]out on the PPMR was also analyzed. Increasing [Ca2+]out from 2 to 8 mM tended to decrease the PPMR in WT neurons (PPMR, 0.97 to 0.91) and Rab3 GEP−/− neurons (1.2 to 1.05); however, these reductions in the PPMR were not significant. The difference in the PPMR between WT and Rab3 GEP−/− neurons was statistically significant in 2 mM [Ca2+]out, but not in 8 mM [Ca2+]out (Fig. 2c). These results suggest that the process affected in Rab3 GEP−/− neurons was rescued to some extent in high [Ca2+]out solution. In the following parts of the paper, 2 mM [Ca2+]out was used.
Analysis of Asynchronous Mini-EPSCs.
The results of the paired-pulse analysis appeared consistent with the hypothesis that the presynaptic site was affected in Rab3 GEP−/− mice; still, this was indirect evidence. To obtain more direct evidence, the amplitude distribution and frequency of asynchronous mini-EPSCs (21) were analyzed in WT and Rab3 GEP−/− neurons (Fig. 3 a1 and a2). The amplitude histogram of all mini-EPSCs showed a skewed distribution with a single peak in both types of neurons (Fig. 3b). The peak value, obtained from amplitude-distribution of each neuron, was considered as the quantal size (q; ref. 22) of each cell. Mean q was 21.9 ± 1.1 pA (n = 9) in WT neurons and 18.9 ± 1.4 pA (n = 7) in Rab3 GEP−/− neurons. Because q was not different between WT and Rab3 GEP−/− neurons (P > 0.1), postsynaptic sensitivity of the AMPA receptor was similar in both types of neurons (Fig. 3c). On the other hand, the frequency of events was significantly higher in WT neurons (34.3 ± 4.4 Hz, n = 9) than in Rab3 GEP−/− neurons (18.3 ± 2.5 Hz, n = 7, P < 0.05) (Fig. 3d). These results clearly indicate that the presynaptic release mechanism is likely to be affected in Rab3 GEP−/− neurons.
Fig 3.
Asynchronous mini-EPSCs. (a) Asynchronous mini-EPSCs recorded in WT neurons (a1, n = 9) and Rab3 GEP−/− neurons (a2, n = 7). (Bars, 50 pA, 0.1 s.) (b) Amplitude distribution of WT neurons (Upper, 1,475 events from 9 cells) and Rab3 GEP−/− neurons (Lower, 670 events from 7 cells). (c) Quantal size (q) in WT neurons (left column, n = 9, total 1,475 events) and in Rab3 GEP−/− neurons (right column, n = 7, total 670 events). (d) Frequency of the asynchronous mini-EPSCs recorded between 0.16 and 0.9 s after the first stimulation in WT neurons (left column, n = 9) and in Rab3 GEP−/− neurons (right column, n = 7). *, P < 0.05.
Different Responses to Repetitive Stimulation in Rab3 GEP−/− Neurons.
Because repetitive stimulation gives us some insights into the nature of the release probability, Pr, and the size of the RRP in the central synapse (23), we stimulated autapses at 20 and 40 Hz in a solution containing 2 mM Ca2+. In WT neurons, the evoked EPSC amplitude decreased gradually during repetitive stimulation at 20 Hz (Fig. 4a1). The baseline gradually shifted inward. Then, both the fast synchronous evoked component and the shift of the baseline reached steady-state levels. In Rab3 GEP−/− neurons, repetitive stimulation caused facilitation in the evoked EPSC during the first several stimuli at 20 Hz (Fig. 4a2), followed by a relatively large steady-state level. The baseline shift was negligible in Rab3 GEP−/− neurons.
Fig 4.
Evoked EPSCs responding to repetitive stimulation. (a) Responses to repetitive stimuli at 20 Hz in WT neurons (a1) and Rab3 GEP−/− neurons (a2). (b) Summary of responses to repetitive stimuli at 20 Hz in WT neurons (triangle, n = 7) and Rab3 GEP−/− neurons (circle, n = 10). (c) Responses to repetitive stimuli at 40 Hz in WT neurons (c1) and Rab3 GEP−/− neurons (c2). Artifacts and spikes are erased in a and c. (Bars, 1 nA, 0.2 s.) (d) Summary of responses to repetitive stimuli at 40 Hz in WT neurons (triangle, n = 7) and Rab3 GEP−/− neurons (circle, n = 10).
Similar properties were observed in evoked responses elicited by repetitive stimulation at 40 Hz. In WT neurons, the fast-evoked response reduced its amplitude on repeated stimulation (Fig. 4c1), and the rate of decrease was higher than that measured by repetitive stimulation at 20 Hz. The fast synchronous component of the evoked response eventually disappeared, whereas the slow asynchronous component remained unchanged as observed at 20-Hz stimulation. In Rab3 GEP−/− neurons, the amplitude of the synchronous evoked component increased during the first 5–10 stimuli, and then slowly decreased to a steady-state level (Fig. 4c2). The baseline shift in Rab3 GEP−/− neurons was much smaller than that in WT neurons as well. Summaries of the responses to 20- and 40-Hz stimulation in 7 WT neurons and 10 Rab3 GEP−/− neurons are shown in Fig. 4 b and d, respectively, which indicates a remarkable difference in time-dependent change in the evoked EPSC amplitude between WT and Rab3 GEP−/− neurons. The slow asynchronous component showed almost no suppression for longer stimulation (longer than 1 s). Thus, there was a remarkable difference between the slow asynchronous and fast synchronous components of the evoked EPSC, and only the latter seemed to be depleted.
Isolation of the Synchronous Component of the Evoked EPSC.
We then attempted to isolate the synchronous evoked component from the asynchronous component. Because the synchronous evoked component decayed within 25 ms during the middle and late phase of repetitive stimulation, the asynchronous component of the previous stimulation was subtracted from each synchronous evoked EPSC as a first approximation. This process may underestimate the synchronous evoked component during the second response to the tenth responses. The summarized profile of the synchronous evoked responses to repetitive stimulation at 40 Hz shows a monotonically decreasing phase and a steady-state phase in WT neurons (n = 7; Fig. 5a). In contrast, the amplitude showed a biphasic profile with a peak before the declining phase and a steady-state phase in Rab3 GEP−/− neurons (n = 10). Because there was cell-to-cell variability in the amplitude of the synchronous component, this simple average of the fast component reflects mostly the properties of those cells that showed the largest responses. Thus, the relative amplitude profile of each cell, normalized by the maximum value of each profile, was averaged in WT and Rab3 GEP−/− neurons (Fig. 5b). This plot clearly outlines a tendency similar to that of the simple average.
Fig 5.
Fast synchronous component of the evoked EPSC elicited by repetitive stimulation at 40 Hz. (a) Simple average of the fast synchronous component of evoked EPSCs in WT neurons (triangle, n = 7) and in Rab3 GEP−/− neurons (circle, n = 10). (b) The mean of the normalized fast synchronous component of evoked EPSCs. In each cell, the fast synchronous component was normalized by the maximum value. In most WT neurons (triangle, n = 7), the first EPSC showed the maximum value. In Rab3 GEP−/− neurons (circle, n = 10), the successive response curve showed a peak at the beginning phase. In both types of neurons, the successive response curve attained the steady-state levels.
Estimation of the RRP Size and the Pr.
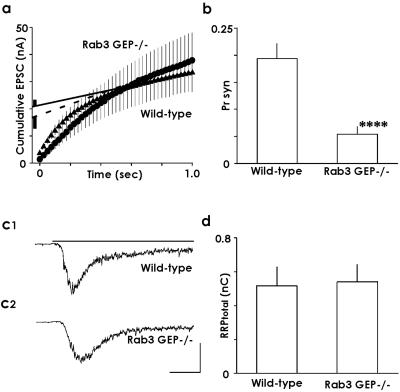
From the cumulative profile of the fast evoked EPSC, the RRP of the synchronous release, which depleted during repetitive stimulation, was roughly estimated by back-extrapolation of its linear portion (0.7–1.0 s, Fig. 6a) to 0 s (23). For this estimation, we assumed that the steady-state level, which corresponded to the gradient of the linear portion of the cumulative profile, indicated an equilibrium level between release and replenishment, and that replenishment was constant during repetitive stimulation. Thus, the back-extrapolated value at the y axis would give the total release minus the total replenishment, which can be viewed as the RRP of the synchronous release measured by repetitive stimulation in this study. This value (RRPsyn) was 21.2 ± 2.4 nA in WT neurons (n = 7) and 16.8 ± 4.0 nA in Rab3 GEP−/− neurons (n = 10). No significant difference was detected between the two types of neurons in the value of RRPsyn. Fig. 6a shows the summary of all recorded cells in both types of neurons. The Pr,syn of the first EPSC was estimated by dividing the first EPSC amplitude by the RRPsyn. The estimated Pr,syn of the first EPSC was significantly reduced in Rab3 GEP−/− neurons (0.05 ± 0.01, n = 10) compared with that in WT neurons (0.19 ± 0.03, n = 7) (Fig. 6b, P < 0.001, t test). The RRP size of the total release (RRPtotal) was estimated by an independent, established method. A hypertonic solution (normal extracellular solution with 500 mM sucrose added; ref. 24) was applied to the autapses, and the charge transfer in the transient part of the induced synaptic current was measured as RRPtotal (Fig. 6 c1 and c2); it was 516 ± 113 pC in WT neurons (n = 4) or 540 ± 102 pC in Rab3 GEP−/− neurons (n = 4; Fig. 6d), and no difference was detected between both types of neurons in the RRPtotal. The Pr,total of the first EPSC was estimated by dividing the charge transfer of the first EPSC by the RRPtotal. The estimated Pr,total was significantly reduced in Rab3 GEP−/− neurons (0.013 ± 0.003, n = 4) compared with that in WT neurons (0.04 ± 0.01, n = 4) (P < 0.005, t test). These data indicate that Pr,total is markedly reduced in Rab3 GEP−/− neurons, whereas the size of RRPtotal is not different between WT and Rab3 GEP−/− neurons.
Fig 6.
Cumulative EPSC amplitude and estimated RRP and Pr. (a) Cumulative EPSC amplitudes during 40 Hz trains in WT neurons (triangle, n = 7) and Rab3 GEP−/− neurons (circle, n = 10). Data points in a range 0.7–1.0 s were fitted by linear regression, and back-extrapolated to 0 s, to estimate the cumulative EPSC amplitudes before steady-state depression, which is assumed to be a first approximation of the RRPsyn. (b) The mean of the estimated value of Pr,syn. The first Pr,syn was obtained by dividing the first EPSC amplitude by the RRPsyn in each cell and averaged. ****, P < 0.0001. (c) Response to hypertonic solution from WT (c1) and Rab3 GEP−/− neurons (c2). Hypertonic solution was applied for 10 s. The first half period was indicated. (Bars, 0.5 nA, 1 s.) (d) Estimated RRPtotal (charge transfer) by hypertonic solution method.
Fine Structure of Autapses of Cultured Hippocampal Neurons.
Our previous studies on the E18.5 Rab3 GEP−/− embryos showed that the number of synaptic vesicles was markedly decreased at the neuromuscular junction, and that the synaptic vesicles were located remote from the presynaptic membrane (16), suggesting that synaptic transmission is primarily impaired in the axonal terminal of the neuromuscular junction. Because our electrophysiological investigations revealed partially intact transmission in autapses of Rab3 GEP−/− hippocampal neurons, at least some synaptic vesicles are expected to be docked on the presynaptic membrane. Electron microscopic analysis did show that synaptic vesicles were present in the presynaptic terminal of the autapse, and that some of the vesicles were actually docked on the active zone of the presynaptic membrane in Rab3 GEP−/− neurons (Fig. 7, n = 6), similar to those in WT neurons (n = 6). However, the mean diameter of Rab3 GEP−/− synaptic vesicles was 40.7 ± 0.8 nm, and this value was smaller than that of WT synaptic vesicles, 50.8 ± 0.7 nm (P < 0.001). In Rab3 GEP−/− synapses, many small synaptic vesicles tended to be located not at the active zones but in the central region of the presynaptic terminals (Fig. 7). Furthermore, immunoreactivity of Rab3A on the synaptic vesicle membrane was markedly decreased in Rab3 GEP−/− neurons (Fig. 7), whereas the distribution of neurofilaments in the presynaptic terminal was similar between WT and Rab3 GEP−/− neurons (data not shown). These morphological changes suggest that the synaptic vesicles in autapses of Rab3 GEP−/− hippocampal neurons are not completely intact although they serve a basic electrophysiological function.
Fig 7.
Fine structure of the autapse. (a and b) Conventional electron microscopy. a, WT; b, Rab3 GEP−/−. (c and d) Rab3A immunoelectron microscopy. c, WT; d, Rab3 GEP−/−. nSV, synaptic vesicles with a normal size; sSV, synaptic vesicles with a smaller size; PSD, postsynaptic density.
Discussion
Our present results reveal a specific function for Rab3 GEP in mouse hippocampal neurons in culture. In Rab3 GEP−/− neurons, the amplitude of the autaptic EPSC was suppressed, the PPMR was enhanced, but the quantal size q was unchanged. These results suggest that the affected site is the presynapse (ref. 25, and see ref. 26). Repetitive stimulation caused different response profiles between WT and Rab3 GEP−/− neurons. WT neurons showed a monotonic depression of responses during repetitive stimulation, like other central synapses (23, 27–29). Desensitization of AMPA receptors cannot be the main cause for the depression, because cyclothiazide, a blocker of AMPA receptor desensitization, does not block this depression (23). Facilitation during repetitive stimulation, observed in Rab3 GEP−/− neurons, is indicative of a reduced release probability Pr (30, 31). This facilitation and the larger PPMR suggest that the Pr is suppressed in Rab3 GEP−/− neurons.
To estimate the size of the RRP approximately by repetitive stimulation, the cumulative EPSC amplitude during repetitive stimulation was calculated (23). For this estimation of the RRPsyn, the slow asynchronous component was subtracted. Although detailed properties of the slow asynchronous component are unknown, it might represent accumulated asynchronous EPSCs (32) or enhanced miniature EPSCs (33). The contribution of spilled-over glutamate during repetitive stimulation could not be excluded; however, RRPsyn sizes estimated by the repetitive stimulation method were not different between WT (966 ± 109 quanta) and Rab3 GEP−/− (887 ± 211 quanta) neurons. The same conclusion was drawn from estimation of the RRPtotal by the hypertonic solution method (Fig. 6; 4,690 ± 1,027 quanta in WT, 5,684 ± 1,074 quanta in Rab3 GEP−/− neurons; ref. 34). In fact, electron microscopy revealed that synaptic vesicles were docked at the presynaptic membrane in Rab3 GEP−/− neurons (Fig. 7). These findings suggest that Rab3 GEP is not involved in the vesicle docking process in the central synapse. Both the Pr,syn and Pr,total were markedly suppressed in Rab3 GEP−/− neurons, which must be the underlying mechanism for the reduction of the EPSC amplitude in Rab3 GEP−/− neurons.
It would be difficult, however, to explain the reduction in Pr of Rab3 GEP−/− neurons by changes in the sensitivity to [Ca2+]out, because the enhanced ratio of the EPSC amplitude elicited by increasing [Ca2+]out from 2 to 8 mM was similar between WT and Rab3 GEP−/− neurons (Fig. 2). This makes a marked contrast between Rab3 GEP−/− neurons and complexin I/II double knocked-out neurons, because the latter showed a severe reduction in Ca2+ sensitivity (29). These two mutants share common features, such as the reduction in the autaptic EPSC amplitude, the facilitatory response to repetitive stimulation, and the normal size of the RRP, except for the difference in Ca2+ sensitivity. An increase in external Ca2+ concentration rescues the evoked EPSC amplitude only slightly (Fig. 2), while Ca2+ influx during repetitive stimulation seems to convert unprimed vesicles into primed ones in Rab3 GEP−/− neurons. Thus, Rab3 GEP is likely to act on a step in the vesicle priming process, rather than in the final fusion step. In contrast, complexin may act downstream to Rab3 GEP function on a step where its action on a single evoked release is sensitive to the increase in external Ca2+.
The functional role of Rab3 GEP shown here, keeping the Pr relatively high, is apparently inconsistent with that of Rab3A, because Rab3A is reported to down-regulate a late step in synaptic vesicle fusion (13). So far, there has been no simple explanation for this difference. One possibility is that the Rab3 GEP deficiency affects functions of Rab3C (35) and -3D (36), as well as Rab3A, that may be involved in the transport of the components regulating the fusion process in neurotransmitter release. Another possibility is that the Rab3 GEP deficiency abolishes functions of proteins that may regulate the postdocking process, thereby masking the loss of Rab3A-mediated function. RIM1α may be one of the candidates. Actually, neurons from RIM1α knockout animals show an increase in the PPMR, facilitation of the response to repetitive stimulation, and a decrease in Pr (37). However, there are differences between the actions of RIM1α and Rab3A in the regulation of synaptic vesicle exocytosis, and it remains unknown how the Rab3 GEP and other proteins act on the postdocking process of synaptic exocytosis cooperatively in the hippocampal region.
We are unable to provide a definitive explanation for the difference in the number of the synaptic vesicles between the central synapse in culture and the peripheral synapse (16) in Rab3 GEP−/− mice. It could be attributed to differential roles of Rab3 GEP in vesicle trafficking between central and peripheral synapses.
Acknowledgments
We thank Prof. E. Neher (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany) for helpful comments on the manuscript. This work is partly supported by grants to K.Y. from Core Research for Evolutional Science and Technology–Japan Science and Technology Corporation, and by grants-in-aid for Scientific Research and for Cancer Research to J.M. and Y.T. from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Abbreviations
EPSC, excitatory postsynaptic current
PPMR, paired-pulse modulation ratio
RRP, readily releasable pool
References
- 1.Takai Y., Sasaki, T., Shirataki, H. & Nakanishi, H. (1996) Genes Cells 1, 615-632. [DOI] [PubMed] [Google Scholar]
- 2.Takai Y., Sasaki, T. & Matozaki, T. (2001) Physiol. Rev. 81, 153-208. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer S. R. (1999) Nat. Cell Biol. 1, E17-E22. [DOI] [PubMed] [Google Scholar]
- 4.Moyer B. D. & Balch, W. E. (2001) Methods Enzymol. 329, 3-6. [DOI] [PubMed] [Google Scholar]
- 5.Zerial M. & McBride, H. (2001) Nat. Rev. Mol. Cell. Biol. 2, 107-117. [DOI] [PubMed] [Google Scholar]
- 6.Wada M., Nakanishi, H., Satoh, A., Hirano, H., Obaishi, H., Matsuura, Y. & Takai, Y. (1997) J. Biol. Chem. 272, 3875-3878. [DOI] [PubMed] [Google Scholar]
- 7.Fukui K., Sasaki, T., Imazumi, K., Matsuura, Y., Nakanishi, H. & Takai, Y. (1997) J. Biol. Chem. 272, 4655-4658. [DOI] [PubMed] [Google Scholar]
- 8.Nagano F., Sasaki, T., Fukui, K., Asakura, T., Imazumi, K. & Takai, Y. (1998) J. Biol. Chem. 273, 24781-24785. [DOI] [PubMed] [Google Scholar]
- 9.Shirataki H., Kaibuchi, K., Sakoda, T., Kishida, S., Yamaguchi, T., Wada, K., Miyazaki, M. & Takai, Y. (1993) Mol. Cell. Biol. 13, 2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Okamoto, M., Schmitz, F., Hofmann, K. & Südhof, T. C. (1997) Nature 388, 593-598. [DOI] [PubMed] [Google Scholar]
- 11.Geppert M., Bolshakov, V. Y., Siegelbaum, S. A., Takei, K., De Camilli, P., Hammer, R. E. & Südhof, T. C. (1994) Nature 369, 493-497. [DOI] [PubMed] [Google Scholar]
- 12.Castillo P. E., Janz, R., Südhof, T. C., Tzounopoulos, T., Malenka, R. C. & Nicoll, R. A. (1997) Nature 388, 590-593. [DOI] [PubMed] [Google Scholar]
- 13.Geppert M., Goda, Y., Stevens, C. F. & Südhof, T. C. (1997) Nature 387, 810-814. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaki H., Miyoshi, J., Kamiya, H., Togawa, A., Tanaka, M., Sasaki, T., Endo, K., Mizoguchi, A., Ozawa, S. & Takai, Y. (2000) Proc. Natl. Acad. Sci. USA 97, 11587-11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Adamo P., Menegon, A., Lo Nigro, C., Grasso, M., Gulisano, M., Tamanini, F., Bienvenu, T., Gedeon, A. K., Oostra, B., Wu, S. K., et al. (1998) Nat. Genet. 19, 134-139. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M., Miyoshi, J., Ishizaki, H., Togawa, A., Ohnishi, K., Endo, K., Matsubara, K., Mizoguchi, A., Nagano, T., Sato, M., et al. (2001) Mol. Biol. Cell 12, 1421-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal M. M. & Furshpan, E. J. (1990) J. Neurophysiol. 64, 1390-1399. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi K., Takada, M., Fujimori, K., Tsuchimoto, Y., Kushima, Y., Sanada, M., Fujiwara, T. & Akagawa, K. (1997) NeuroReport 8, 3641-3644. [DOI] [PubMed] [Google Scholar]
- 19.Hamill O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. (1981) Pflügers Arch. 391, 85-100. [DOI] [PubMed] [Google Scholar]
- 20.Mizoguchi A., Nakanishi, H., Kimura, K., Matsubara, K., Ozaki-Kuroda, K., Katata, T., Honda, T., Kiyohara, Y., Heo, K., Higashi, M., et al. (2002) J. Cell Biol. 156, 555-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliet S. H. R., Malenka, R. C. & Nicoll, R. A. (1996) Science 271, 1294-1297. [DOI] [PubMed] [Google Scholar]
- 22.Redman S. (1990) Physiol. Rev. 70, 165-198. [DOI] [PubMed] [Google Scholar]
- 23.Schneggenburger R., Meyer, A. C. & Neher, E. (1999) Neuron 23, 399-409. [DOI] [PubMed] [Google Scholar]
- 24.Rosenmund C. & Stevens, C. F. (1996) Neuron 16, 1197-1207. [DOI] [PubMed] [Google Scholar]
- 25.Oleskevich S., Clements, J. & Walmsley, B. (2000) J. Physiol. 524, 513-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zucker R. S. & Regehr, W. G. (2002) Annu. Rev. Physiol. 64, 355-405. [DOI] [PubMed] [Google Scholar]
- 27.Oleskevich S. & Walmsley, B. (2000) J. Physiol. 526, 349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-Chacón R., Königstorfer, A., Gerber, S. H., Garcia, J., Matos, M. F., Stevens, C. F., Brose, N., Rizo, J., Rosenmund, C. & Südhof, T. C. (2001) Nature 410, 41-49. [DOI] [PubMed] [Google Scholar]
- 29.Reim K., Mansour, M., Varoqueaux, F., McMahon, H. T., Südhof, T. C., Brose, N. & Rosenmund, C. (2001) Cell 104, 71-81. [DOI] [PubMed] [Google Scholar]
- 30.Zucker R. S. (1989) Annu. Rev. Neurosci. 12, 13-31. [DOI] [PubMed] [Google Scholar]
- 31.Thomson A. M. (2000) Trends Neurosci. 23, 305-312. [DOI] [PubMed] [Google Scholar]
- 32.Goda Y. & Stevens, C. F. (1994) Proc. Natl. Acad. Sci. USA 91, 12942-12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narita K., Akita, T., Osanai, M., Shirasaki, T., Kijima, H. & Kuba, K. (1998) J. Gen. Physiol. 112, 593-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens C. F. & Tsujimoto, T. (1995) Proc. Natl. Acad. Sci. USA 92, 846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer von Mollard G., Stahl, B., Khokhlatchev, A., Südhof, T. C. & Jahn, R. (1994) J. Biol. Chem. 269, 10971-10974. [PubMed] [Google Scholar]
- 36.Adachi R., Nigam, R., Tuvim, M. J., DeMayo, F. & Dickey, B. F. (2000) Biochem. Biophys. Res. Commun. 273, 877-883. [DOI] [PubMed] [Google Scholar]
- 37.Schoch S., Castillo, P. E., Jo, T., Mukherjee, K., Geppert, M., Wang, Y., Schmitz, F., Melenka, R. C. & Südhof, T. C. (2002) Nature 415, 321-326. [DOI] [PubMed] [Google Scholar]