Abstract
High levels of d-serine occur in the brain, challenging the notion that d-amino acids would not be present or play a role in mammals. d-serine levels in the brain are even higher than many l-amino acids, such as asparagine, valine, isoleucine, and tryptophan, among others. d-serine is synthesized by a serine racemase (SR) enzyme, which directly converts l- to d-serine. We now report that SR is a bifunctional enzyme, producing both d-serine and pyruvate in cultured cells and in vitro. Transfection of SR into HEK 293 cells elicits synthesis of d-serine and augmented release of pyruvate to culture media. We identified substances present in HEK 293 and astrocyte cell extracts that strongly stimulate d-serine production by SR and elicit production of pyruvate. Experiments with recombinant enzyme reveal that Mg2+ and ATP present in the cell extracts are physiological cofactors and increase 5- to 10-fold the rates of racemization and production of pyruvate. As much as three molecules of pyruvate are synthesized for each molecule of d-serine produced by SR. This finding constitutes a previously undescribed mechanism underlying d-amino acid synthesis in mammals, different from classical amino acid racemases present in bacteria. Our data link the production of d-serine to the energy metabolism, with implications for the metabolic and transmitter crosstalk between glia and neurons.
Keywords: glutamate receptors, pyruvate, astrocytes
In recent years, it has been shown that astrocytes and neurons exhibit a dynamic bidirectional signaling that profoundly influences neuronal activity and development (1). Astrocytes modulate synaptic transmission by releasing chemical transmitters and eliciting Ca2+ waves in nearby neurons. The search for new transmitter molecules in the brain uncovered the presence of high levels of D-serine in astrocytes, a D-amino acid not previously thought to occur in mammals (2, 3). D-serine is synthesized by a glial-enriched enzyme serine racemase (SR), which directly converts L-to D-serine (4–7). SR does not bear significant homology with bacterial racemases, and little is known about the mechanism and regulation of D-serine production.
D-serine released from astrocytes seems to be an endogenous ligand of the N-methyl-D-aspartate (NMDA) receptor (3, 8). Depletion of endogenous D-serine in slices and cultured cells strongly diminishes NMDA receptor responses measured biochemically and electrophysiologically (8). Massive stimulation of NMDA receptors is implicated in neural damage after stroke (9), and inhibitors of SR may be useful to prevent stroke damage. Thus, inhibitors of SR provide a strategy to decrease NMDA receptor coactivation and may be useful in conditions in which overstimulation of NMDA receptors plays a pathological role.
To clarify the role of D-serine as a modulator of NMDA receptors, one should identify the factors that regulate SR and D-serine signaling. In this paper, we explored mechanisms regulating the production of D-serine by SR. We demonstrate that SR is a unique bifunctional enzyme, producing D-serine and pyruvate at the same time. This property was revealed by our identification of cellular cofactors of the enzyme. We found that Mg2+and ATP are physiological ligands of SR that increase severalfold the production of D-serine and elicit considerable pyruvate generation by direct deamination of L-serine. Our present data reveal a link between D-amino acid synthesis and a new metabolic route for production of pyruvate in the brain.
Materials and Methods
Materials.
Adenine nucleotides, L- and D-amino acids, aminooxyacetic acid, apyrase (grade VII), catalase, glutathione-agarose, NADH, pyridoxal 5′-phosphate (PLP), and Tris were obtained from Sigma. DMEM, FBS, glutamine, penicillin–streptomycin, and trypsin solutions were obtained from Biological Industries (Kibbutz Beit Haemek, Israel). D-amino acid oxidase from pig kidney (EC 1.4.3.3) and lactate dehydrogenase were obtained from Roche Molecular Biochemicals. L-[14C(U)]serine was purchased from Perkin–Elmer. Thrombin cleavage capture kit was purchased from Novagen. Other reagents were of analytical grade.
Stable Expression in HEK 293 Cells.
Mouse full-length SR was subcloned into pCMV-GST expression vector containing a cytomegalovirus promoter and the gene for the GST of Schistosoma japonicum (6). The construct was cotransfected into HEK 293 cells with a plasmid containing the gene for resistance to neomycin (PIRESneo, CLONTECH). Resistant clones were selected by using 400 μg/ml G418 (Life Technologies, Grand Island, NY), and several independent colonies were isolated and expanded. Clones expressing highest levels of SR were selected by Western blot analysis using a polyclonal antibody against SR. The cells were maintained in DMEM plus 10% FBS supplemented with 200 μg/ml G418.
Purification of Recombinant SR.
Stable HEK 293 cell line expressing SR-GST was cultured in DMEM plus 10% FBS. Cells grown in 140-mm dishes were harvested and lysed in medium containing 20 mM Tris⋅HCl (pH 7.4), 500 mM NaCl, 1% Triton X-100, 0.2 mM PMSF, 2 mM EDTA, 2 mM DTT, and 15 μM PLP. The lysate was centrifuged at 40,000 × g for 20 min at 4°C to remove insoluble material. The supernatant was incubated overnight under slow rotation at 4°C with glutathione-agarose beads to bind SR-GST fusion protein. The beads were washed six times with PBS supplemented with 0.5% Triton X-100/300 mM NaCl/15 μM PLP. The beads were subsequently washed four times with PBS plus 15 μM PLP to remove the salt and detergent. SR-GST fusion protein was eluted by batch incubation with 40 mM Tris⋅HCl (pH 8.5), 10–20 mM reduced glutathione, 15 μM PLP, and 500 mM NaCl. The eluate was dialyzed for 2 h against PBS plus 15 μM PLP. This preparation was used for most experiments. Typically, 0.8–1.4 mg of SR-GST was obtained from 40 confluent HEK 293 culture dishes of 140-mm diameter. In some experiments, the GST part was removed by incubating beads containing SR-GST with biotinylated thrombin for 16 h at room temperature in PBS plus 15 μM PLP. Purified SR was obtained after GST and biotinylated thrombin were removed with glutathione-agarose and streptavidin-agarose beads, respectively. The very high purity of this preparation has been described elsewhere (6).
Cell Transfection.
HEK 293 cells were split into 6-well tissue culture plates (Nunc) at 70–90% confluence. On the next day, cells were transfected with 0.4 μg of mouse full-length SR gene, a catalytically inactive mutant constructed by replacing Lys-56 with glycine (5) or GFP cloned into pRK5-KS by using Effectene reagent (Qiagen, Valencia, CA). Cells were used for experiments 2 days after transfection. Cells transfected with GFP revealed ≈30% transfection efficiency monitored by fluorescence activated cell sorting (FACSCalibur, Becton Dickinson).
Assay of d- and l-Serine in Cells.
HEK 293-transfected cells were incubated in 6-well plates with 0.8 ml of DMEM, which contains about 0.6 mM L-serine and 6 μM contaminating D-serine. To calculate specific synthesis of D-serine, the values of contaminating D-serine in DMEM were subtracted from the values obtained after addition of 10 mM L-serine. To measure L- and D-serine, a 0.1-ml aliquot of culture medium was removed, and the samples were processed for HPLC as described (10). Briefly, samples were first deproteinized by addition of trichloroacetic acid (TCA) at 5% final concentration. The suspension was centrifuged at 20,000 × g for 5 min, and the supernatant was analyzed by HPLC after removal of TCA by four extractions with water-saturated diethyl ether. For intracellular amino acid determination, the transfected cells were washed twice with cold PBS, followed by the addition of 5% TCA to extract free amino acids. The suspension was centrifuged at 20,000 × g for 5 min, and the supernatant was analyzed.
Assay of Pyruvate.
To monitor pyruvate levels, a 0.1-ml aliquot of culture medium was removed and boiled for 5 min to inactivate endogenous lactate dehydrogenase activity. The samples were centrifuged at 20,000 × g for 10 min to remove precipitated protein, and the supernatant was analyzed for pyruvate by monitoring the decrease in NADH (0.2 mM) absorbance at 340 nm, as the pyruvate was converted to lactate by added lactate dehydrogenase (1 μg/ml) (11). To determine the synthesis of [14C]pyruvate, transfected cells were incubated for 12 h with a 1-ml culture medium containing 10 mM L-[14C(U)]serine (0.5–2 μCi per 3.5 cm well; 1 Ci = 37 GBq). Pyruvate was separated from labeled serine by pouring the TCA-deproteinized and ether-extracted culture media (adjusted to pH 6.0) onto a 1-ml anion exchange column (Dowex 1X4, 200–400 mesh, Cl form, Supelco), which bound all pyruvate but not serine. The column was washed with 5 ml of water, and [14C]pyruvate was eluted with 2 ml of 2 M HCl. To determine the extent of pyruvate labeling, the eluate was derivatized with 2-nitrophenylhydrazine (Sigma) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma) and chromatographed on a reverse-phase column with acetonitrile/methanol/water (30:20:50, vol/vol/vol) as the eluent (12). The peak corresponding to pyruvate was collected, and the radioactivity was determined by scintillation counting. The recovery of pyruvate by HPLC was about 60% as revealed by a standard containing known amount of [14C]pyruvate. Blanks were made with media containing 10 mM L-[14C(U)]serine that were not exposed to cells.
Production of Deproteinized Cell Extracts.
Confluent HEK 293 cells cultured in 6-well plates were washed twice with cold PBS. The cell content of one well was scraped off the plate and transferred to a 1.5-ml tube on ice. The suspension was centrifuged for 15 s at 20,000 × g, and the supernatant was discarded. The cell pellet was suspended in 0.1 ml of cold PBS, and the protein was removed by centrifugation after precipitation with 5% TCA. The acid was further extracted with diethyl ether, and the aqueous phase containing soluble nonproteinaceous molecules was neutralized with NaOH. The samples were centrifuged at vacuum for 5 min to remove any residual diethyl ether. For deproteinized extracts from astrocytes, we prepared primary astrocyte cultures in 6-well plates as described (13). After 14 days in culture, the cells were scraped off the plate, and the protein was removed by TCA precipitation as described above for HEK 293 extracts.
Activity Assays.
Reaction media contained 20 mM Tris⋅HCl (pH 7.4), 15 μM PLP, and 10 mM L-serine in a final reaction volume of 60 μl. The reaction was started by addition of recombinant SR (0.15 mg/ml in PBS) and stopped after 1–2 h at 37°C by boiling for 5 min. Blanks were carried out with heat-inactivated enzyme. To test the effects of deproteinized cell extracts on SR activity, the reaction was carried out in the presence of 30 μl of extracts, keeping the final reaction volume at 60 μl. D-serine was monitored by HPLC analysis. Formation of pyruvate from L-serine was monitored with lactate dehydrogenase as described above. When indicated, the free Mg2+ concentration was varied by using an Mg–EDTA buffer. The concentration of EDTA was fixed at 2 mM, and the amounts of MgCl2 needed to obtain the desired free Mg2+ concentrations were calculated by using Mg–EDTA and MgATP association constants previously determined (14). To remove contaminating D-serine, which accounts for 1% of total serine in commercial preparation, a 50 mM L-serine solution in 20 mM Tris⋅HCl (pH 8.8) was incubated with 30 units of D-amino acid oxidase and 500 units of catalase for 48 h at 37°C. At the end of the incubation, the solution was boiled for 5 min, and the denatured protein was removed by centrifugation at 10,000 × g for 10 min. The pH of the solution was adjusted to 6.0, and the hydroxypyruvate generated by degradation of D-serine was removed by passing the solution through a 5-ml column of Dowex 1X4 anion exchange resin (Supelco). Then, the pH was adjusted to 7.4 with NaOH, and the cleaned L-serine was concentrated to 300 mM under vacuum. Treated L-serine contained <0.3% contaminant D-serine and no detectable hydroxyopyruvate monitored by lactate dehydrogenase method.
Apyrase Treatment.
To deplete endogenous ATP, HEK 293 cell extracts were incubated for 10 min at 37°C with 10 units of apyrase. Controls were made with apyrase inactivated by a 10-min preincubation at 100°C.
ATPase Activity.
ATPase activity was assayed by measuring the release of 32Pi from [γ-32P]ATP as described (15). Reaction medium contained 20 mM Tris⋅HCl (pH 7.4), 100 μM [γ-32P]ATP, 1 mM MgCl2, 15 μM PLP, 10 mM L-serine, 0.1 mg/ml SR. The reaction was stopped after 2 h at 37°C. Blanks were carried out with heat-inactivated SR.
ATP Determination.
ATP level was determined by luciferase-driven bioluminescence using the ATP bioluminescence assay kit CLS II (Roche Biochemicals). Luminescence was monitored with a microplate luminometer (Anthos Lucy 1).
Ammonia Determination.
Ammonia produced by deamination of L-serine was monitored in a 10-μl aliquot of the reaction medium. NH3 was determined by the oxidation of NADPH in the presence of 2-oxolutarate and glutamate dehydrogenase (ammonia kit, Sigma).
Results
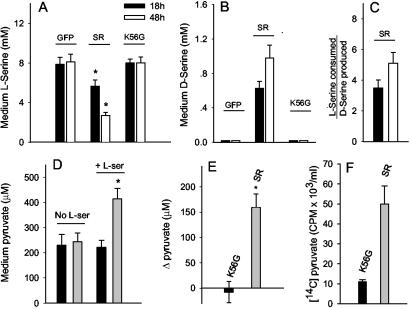
We sought to look for factors regulating SR-driven D-serine production in cells. Transfection of HEK 293 cells with SR gene elicits a decrease in medium L-serine, accompanied by an increase in medium D-serine to values as high as 1 mM (Fig. 1 A and B). Transfection with a control gene (GFP) or a catalytically inactive mutant (K56G) has no effect on L-serine levels and does not elicit D-serine synthesis. The same qualitative changes are observed when the intracellular amino acid levels are analyzed, indicating that the medium levels of serine are in equilibrium with and faithfully reflect intracellular levels (data not shown).
Fig 1.
L-serine consumption, D-serine synthesis, and pyruvate production in transfected cells. HEK 293 cells were transfected with GFP, SR, or an inactive mutant of SR (K56G). (A) Medium levels of L-serine decrease in SR-transfected cells, both 18 or 48 h after addition of L-serine to media. (B) Synthesis of D-serine in SR-transfected cells. Note that the scales of A and B are different. (C) Ratio of L-serine consumed to D-serine produced. A larger than expected decrease in medium L-serine was observed when compared with the amount of D-serine synthesized. (D) Pyruvate in culture media was analyzed 18 h after addition of none (No L-ser) or 10 mM L-serine (+ L-ser). (E) Quantification of pyruvate changes in K56G and SR. The values of pyruvate without added L-serine were subtracted from the values with L-serine and are expressed as Δpyruvate. (F) Augmented formation of [14C]pyruvate from L-[14C]serine in culture medium of SR-transfected cells. The results represent the average ± SEM of four (A–C), nine (D and E), or three (F) independent transfection experiments carried out in triplicates. *, Different from control at P < 0.050 (Student's t test).
We observed that the decrease in L-serine was more pronounced than the total synthesis of D-serine, i.e., about 4 mol of L-serine are consumed for each mol of D-serine produced (Fig. 1C). This gap is not caused by degradation of produced D-serine by the cells, as HEK 293 cells do not possess significant amounts of D-amino acid oxidase. Thus, addition of 1 mM D-serine to HEK 293 cultured cells does not result in significant degradation even after 48 h of incubation (data not shown). This finding suggests that L-serine is converted to an additional compound in the cells, as if SR would be able to catalyze other types of reactions toward L-serine.
The lack of significant alterations in other amino acid levels present in the culture medium led us to investigate whether L-serine might be converted by the cells to a nonamino acid, such as pyruvate. We found that addition of L-serine to cells transfected with SR elicits a considerable increase in pyruvate concentration in the culture medium (≈160 μM, Fig. 1 D and E). By contrast, addition of L-serine to cultures transfected with catalytically inactive mutant (K56G) does not increase pyruvate levels (Fig. 1E). Conversion of L-serine into pyruvate was monitored by adding L-[14C]serine to culture medium and analyzing pyruvate by HPLC. A 5-fold higher specific labeling of pyruvate is detected in SR-transfected cells when compared with control culture (Fig. 1F).
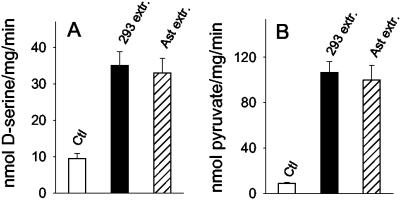
The larger formation of labeled pyruvate in SR-transfected cultures suggests that SR might physiologically produce pyruvate from L-serine. Nevertheless, our previous attempts to detect pyruvate production from L-serine have failed when we used brain-purified or recombinant SR. Thus, we hypothesized that the enzyme requires additional cofactors to catalyze deamination of L-serine to pyruvate. We prepared deproteinized extracts from HEK 293 cells and primary astrocyte cultures and tested their effects on D-serine synthesis and pyruvate production by the recombinant purified SR. Accordingly, addition of deproteinized cell extracts leads to a 4-fold activation of D-serine synthesis by SR (Fig. 2A). The cell extracts mimic the effects observed in transfected cells because they also elicit robust production of pyruvate from L-serine catalyzed by SR (Fig. 2B). This demonstrates that SR is capable of direct conversion of L-serine into pyruvate. The activators present in the cell extracts are very potent because the degree of activation remains unchanged when the extracts are diluted 10-fold in PBS (data not shown). Similar activation is detected when extracts of brain and liver are added (data not shown). The ability of SR to catalyze pyruvate production would be otherwise unnoticed without the stimulation elicited by the cell extracts because the basal production of pyruvate is close to the detection limit of the method used.
Fig 2.
Stimulatory effect of deproteinized cell extracts on SR. Deproteinized extracts from HEK 293 (293 extr.) and primary astrocyte (Ast. extr.) cultured cells elicit an increase in D-serine synthesis (A) and pyruvate production (B) by recombinant SR. Reaction media contained 20 mM Tris⋅HCl (pH 7.4), 15 μM PLP, and 10 mM L-serine and 0.15 mg/ml of recombinant SR-GST. The reaction was stopped after 1 h at 37°C, and the samples were assayed for D-serine and pyruvate. The results represent the average ± SEM of three to five experiments carried out with different protein preparations.
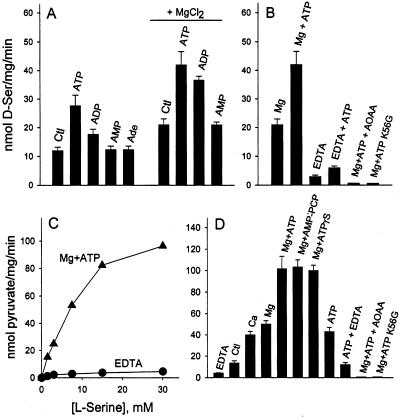
To identify the nature of the factor(s) present in the cellular extracts, we tested the effect of a variety of ubiquitous molecules on the racemization of L-serine by recombinant SR. We found that production of D-serine is stimulated by addition of ATP and to a lesser extent by ADP, whereas AMP has no effect (Fig. 3A). Because the complex MgATP is a high-affinity ligand of numerous ATP binding enzymes, we tested whether Mg2+ potentiates the stimulation by ATP. Accordingly, addition of Mg2+ increases both the basal and ATP-stimulated D-serine production. The combined effect of Mg2+ and ATP leads to a 5-fold increase in D-serine production compared with control (Fig. 3B). Omission of Mg2+ and chelation of contaminating divalent cations with EDTA strongly inhibits D-serine synthesis (Fig. 3B).
Fig 3.
Stimulation of D-serine and pyruvate production by Mg2+ and adenine nucleotides. (A) Addition of 1 mM ATP and, to a lesser extent, ADP increases the rate of D-serine synthesis. AMP and adenosine (Ade) have no effect. MgCl2 (1 mM) increases the rate of D-serine synthesis both in the absence and in the presence of nucleotides. (B) EDTA (1 mM) inhibits the racemization both in the absence and in the presence of 1 mM ATP. Synthesis of D-serine is abolished by 1 mM aminooxyacetic acid, an inhibitor of PLP-dependent enzymes. No activity is observed with the inactive mutant K56G. (C) Representative experiment showing L-serine concentration dependence of pyruvate production in the presence of 1 mM MgCl2 and 1 mM ATP. Very little pyruvate is detectable in the presence of EDTA. (D) Pyruvate production is stimulated by 1 mM CaCl2, 1 mM MgCl2, alone or in combination with 0.1 mM AMP-PCP or ATPγS. Pyruvate synthesis in the presence of 1 mM ATP alone is inhibited by EDTA, indicating a role of divalent cations in association with ATP. Production of pyruvate is abolished by 1 mM aminooxyacetic acid or when K56G inactive mutant is substituted for SR. The results represent the average ± SEM of four experiments carried out with different protein preparations.
Mg2+ and ATP mimic the effects of the cellular extracts on SR by also stimulating pyruvate production (Fig. 3C). The L-serine concentration dependence for pyruvate production (Fig. 3C, Km = 8 mM) is very similar to that previously reported for D-serine synthesis (4). EDTA, a divalent cation chelating agent, strongly inhibits pyruvate production (Fig. 3C). Addition of Mg2+ or Ca2+ increases pyruvate synthesis (Fig. 3D), and their effects are not additive (date not shown), suggesting that they share the same binding site. EDTA inhibits the basal pyruvate production, indicating a certain degree of activation of SR by contaminating cations present in the reaction medium (Fig. 3D).
To rule out nonspecific effects caused by the GST part of the fusion protein, we removed the tag with thrombin, generating pure SR. The stimulation promoted by cellular extracts and Mg2+ + ATP is identical with SR lacking the GST part (data not shown).
Stimulation by ATP does not require hydrolysis because the nonhydrolyzable analogs, adenosine 5′-[γ-thio]triphosphate and AMP-PCP, are also equally effective in stimulating SR (Fig. 3D). We also did not detect specific hydrolysis of [γ-32P]ATP by SR under our experimental conditions (data not shown). ATP alone stimulates SR, an effect that is diminished by EDTA, indicating that contaminating divalent cations potentiate the effect of ATP (Fig. 3D). The generation of pyruvate and D-serine is not caused by contaminating enzymes in our preparation. Both reactions are abolished by aminooxyacetic acid, an inhibitor of PLP-dependent enzymes, and are absent when K56G inactive mutant is substituted for SR (Fig. 3 B and D).
To directly determine the deamination of L-serine into pyruvate, we monitored NH3 production by the oxidation of NADPH in the presence of 2-oxolutarate and glutamate dehydrogenase. We found that the levels of NH3 produced correspond stoichiometrically to the amount of pyruvate (data not shown).
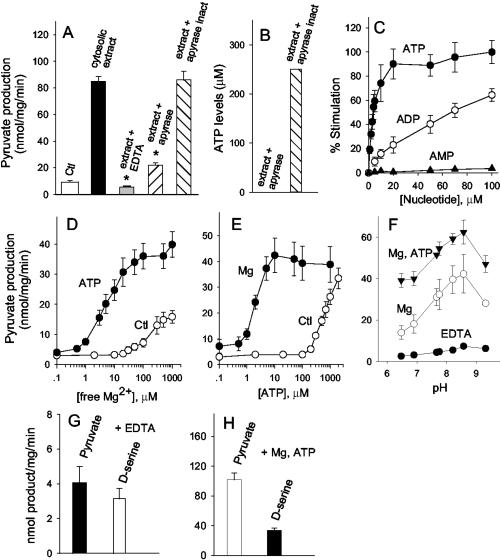
To investigate whether ATP present in HEK 293 extract is the major regulator of SR, we treated the cell extract with purified apyrase, which is known to hydrolyze the γ and β phosphates of ATP, generating AMP. Accordingly, a 10-min preincubation with apyrase significantly diminishes the stimulatory effect of cellular extract on pyruvate production (Fig. 4A). Heat-inactivated apyrase has no effect (Fig. 4A). Levels of endogenous ATP in the cellular extract are about 250 μM, and apyrase treatment decreases it to about 20 nM (Fig. 4B). Activation by cell extract is abolished by EDTA, indicating that divalent cations, such as Mg2+, are required in association with ATP (Fig. 4A).
Fig 4.
Identification of ATP and divalent cations as physiological cofactors present in cell extracts. (A) Previous treatment of HEK 293 cell extract with apyrase strongly decreases the stimulation of pyruvate production by the extract (P < 0.05). Heat-inactivated apyrase has no effect. Chelation of divalent cations with 1 mM EDTA also abolishes the activation by cellular extract (P < 0.05). (B) A representative measurement of ATP concentration in HEK 293 cell extract shows that treatment with apyrase diminishes ATP levels to 20 nM. (C) Nucleotide concentration dependence for the activation of pyruvate production by SR. Reaction was carried out as in legend to Fig. 2, except that it contained 1 mM MgCl2. The values are expressed as percent of stimulation of pyruvate production. (D) ATP potentiates Mg2+ effect. Free Mg2+ was varied in the absence (○) or in the presence (•) of 100 μM ATP using an Mg–EDTA buffer. (E) The activation promoted by ATP in the presence of EDTA (○) was potentiated by addition of MgCl2 at 100 μM free Mg2+ concentration (•). (F) pH profile of pyruvate production in the presence of EDTA (•), 1 mM MgCl2 (○), or 1 mM MgCl2 plus 1 mM ATP (▾). (G) Stoichiometry of pyruvate and D-serine in the presence of 1 mM EDTA. A higher protein concentration (0.6 mg SR-GST/ml) was used to allow easier detection of pyruvate. Other conditions were as in Fig. 2. (H) In the presence of 1 mM MgCl2 and ATP, about 3 molecules of pyruvate are synthesized for each D-serine. Note that the scales in G and H are different. The results represent the average ± SEM of three (A–E), four (F), nine (G), and eight (H) experiments carried out with different protein preparations.
The affinity of SR to ATP is very high (Km = 3 μM), about 30-fold higher than for ADP (Km = 100 μM) (Fig. 4C). Although apyrase enzyme also degrades ADP, the lower levels of intracellular ADP (16) and the much lower affinity of SR to ADP indicate that ADP is not likely to physiologically regulate SR. Other nucleotides, such as GTP, ITP, UTP, and CTP, also stimulate SR (data not shown). Like ADP, however, they are at least one order of magnitude less potent than ATP, supporting the notion that ATP is the main nucleotide cofactor of SR.
We observed a synergy between Mg2+ and ATP effects, supporting a role for an Mg–ATP complex in stimulating SR (Fig. 4). When Mg2+ and ATP are combined, micromolar amounts are enough to fully activate SR, contrasting to the 50- to 100-fold higher concentrations required when each cofactor is present alone (Fig. 4 D and E). Stimulation of pyruvate production is observed at a wide range of pH values, with maximal activity at pH 8.5, a pH value that also supports the highest racemization activity toward L-serine (4).
We determined the ratio of pyruvate/D-serine production. With EDTA, very low amounts of pyruvate and D-serine are produced at similar rates (Fig. 4G). In the presence of Mg2+ and ATP, pyruvate production is more pronounced than D-serine synthesis, so that 3 molecules of pyruvate are produced for each molecule of D-serine (Fig. 4H). This finding explains the substantial release of pyruvate observed in HEK 293 cells transfected with SR and establishes a mechanism underlying D-amino acid production in mammalian brain.
Discussion
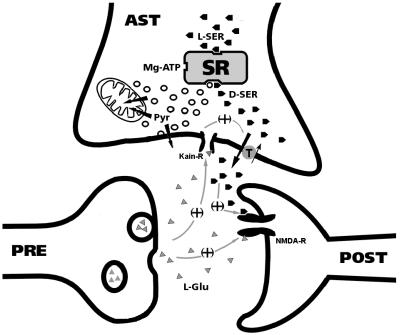
So far, D-amino acid production in mammals has not been linked to any known metabolic route. Our results now indicate that D-serine synthesis is closely linked to energy metabolism of the cells and reveal a putative metabolic pathway for production of pyruvate in the brain (Fig. 5). Pyruvate is a strong neuroprotectant in animal models of stroke, also protecting cells against oxidative damage and zinc neurotoxicity (17–19). Thus, SR-derived pyruvate is likely to play important roles in astrocytes. It is not clear, however, whether SR-derived pyruvate is eventually exported to neurons and capable of altering their ability to deal with NMDA receptor activation promoted by SR-derived D-serine (Fig. 5).
Fig 5.
Proposed roles of D-serine and pyruvate in glial-neuronal communication. D-serine and pyruvate are synthesized from L-serine in astrocytes (AST) by SR. Glutamate (L-Glu) released from neurons interacts with kainate-type of glutamate receptors (Kain-R) in astrocytes to stimulate release of D-serine (3, 13). D-serine acts in concert with L-glutamate (triangles) to activate NMDA receptors (8). Both release of D-serine and the termination of D-serine signaling may be carried out by a neutral amino acid transporter that transports D-serine (13). Pyruvate synthesized by SR may be either used by mitochondria as an energy fuel to cope with the astrocytic metabolic demands during augmented neurotransmission or be released from the cells as pyruvate or lactate.
We have demonstrated that SR is a bifunctional enzyme that catalyzes racemization of L-serine by a previously undescribed mechanism. Our results indicate that D-amino acid racemization in mammalian brain is not related to the conservation of classical bacterial or fungal amino acid racemases to which SR has no homology. Production of pyruvate by SR is reminiscent of its homology with serine dehydratases of Escherichia coli (36% overall identity) and with rat serine dehydratase (28% identity), implying that SR shares a common heritage with serine dehydratase, but acquired a unique capability of catalyzing racemization. Occurrence of racemization in the course of dehydration/deamination reaction has not been observed for any other enzyme. This activity can be explained by the numerous transformations of amino acids that are carried out by pyridoxal 5′-phosphate enzymes; the specific reaction depends on the microenvironment of the catalytic site (20). The bifunctional role of SR explains our previous finding that SR generates pyruvate from L-serine O-sulfate, an artificial L-serine analog and inhibitor of D-serine synthesis by SR (6). The properties we describe for SR should help the identification of other racemases in mammals, such as the enzyme responsible for D-aspartate synthesis in primary neuronal cultures (21).
Mg2+ and ATP activate SR at concentrations much below those found in the cytosol, which are about 0.6 and 3–6 mM, respectively (16, 22). The content of ATP in normal cellular extracts (≈250 μM) is in large excess to that needed to activate SR, explaining our observation that a 10-fold dilution does not affect their stimulatory activity. Only a 100-fold dilution of the cellular extracts effectively diminished the stimulation by decreasing the ATP levels below the Km of the enzyme. Even after stroke and cellular ischemia, intracellular ATP is higher than the levels required to fully activate SR. Hence, studies in animal models showed an increase in D-serine release in extracellular fluid after stroke, which might have a deleterious effect by enhancing NMDA receptor-mediated neurotoxicity (23).
So far, SR properties have been studied only at nonphysiological alkaline pH because of the low SR activity at neutral pH and the difficulties in routine detection of D-serine (4, 24). Also, the inclusion of EDTA in earlier studies precluded the detection of pyruvate production by SR (4). The activation of SR by Mg2+ and ATP we described will allow the study of SR function at physiological relevant conditions, by using a routine assay for the detection of pyruvate.
Cook et al. (24) recently showed that Ca2+ binds to SR and proposed that the cation is a major regulator of SR. Half-maximal stimulation of D-serine synthesis by Ca2+ was observed at 26 μM, which is at least two orders of magnitude higher than the resting concentration in the cells. Although our data do not rule out an effect of Ca2+, it points to a more prominent role of Mg2+, as the concentration of Mg2+ that stimulates SR in the presence of ATP is at least 30-fold lower than the intracellular free Mg2+ (22).
It has been shown that the closest homolog of SR, the catabolic serine dehydratase of E. coli, is activated by AMP, but the effect does not require cations, and the enzyme also does not catalyze racemization (25). The ATP binding region in SR is not obvious, because careful Prosite, Pfam, Profile Scan, and Blocks program analysis for consensus sequences was unable to identify significant homologies to canonical ATP binding sequences. Identification of ATP binding region in SR and determination of the protein structure should clarify the molecular mechanism of ATP action.
Acknowledgments
We thank Simone Leao and Paulo Henrique dos Santos for technical assistance. H.W. thanks Profs. A. Hershko, M. Fry, M. Gavish, and A. Ciechanover for encouragement and kind support, and Dr. M. D. Toney for helpful discussions. This work was supported by research grants from the Theodore and Vada Stanley Foundation, the Fund for the Promotion of Research at the Technion, and the A. and E. Blum Medical Research Fund. J.d.M. and R.P. are recipients of a predoctoral fellowship from Conselho Nacional de Pesquisas (Brazil). H.W. is recipient of a WD/ Irving and Adele Rosenberg Family Academic Lectureship.
Abbreviations
NMDA, N-methyl-d-aspartate
PLP, pyridoxal 5′-phosphate
SR, serine racemase
TCA, trichloroacetic acid
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Haydon P. G. (2001) Nat. Rev. Neurosci. 2, 185-193. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto A., Nishikawa, T., Oka, T. & Takahashi, K. (1993) J. Neurochem. 60, 783-786. [DOI] [PubMed] [Google Scholar]
- 3.Schell M. J., Molliver, M. E. & Snyder, S. H. (1995) Proc. Natl. Acad. Sci. USA 92, 3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolosker H., Sheth, K. N., Takahashi, M., Mothet, J. P., Brady, R. O., Jr., Ferris, C. D. & Snyder, S. H. (1999) Proc. Natl. Acad. Sci. USA 96, 721-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolosker H., Blackshaw, S. & Snyder, S. H. (1999) Proc. Natl. Acad. Sci. USA 96, 13409-13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panizzutti R., de Miranda, J., Ribeiro, C. S., Engelender, S. & Wolosker, H. (2001) Proc. Natl. Acad. Sci. USA 98, 5294-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Miranda J., Santoro, A., Engelender, S. & Wolosker, H. (2000) Gene 256, 183-188. [DOI] [PubMed] [Google Scholar]
- 8.Mothet J. P., Parent, A. T., Wolosker, H., Brady, R. O., Jr., Linden, D. J., Ferris, C. D., Rogawski, M. A. & Snyder, S. H. (2000) Proc. Natl. Acad. Sci. USA 97, 4926-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi D. W. & Rothman, S. M. (1990) Annu. Rev. Neurosci. 13, 171-182. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto A., Nishikawa, T., Oka, T., Takahashi, K. & Hayashi, T. (1992) J. Chromatogr. 582, 41-48. [DOI] [PubMed] [Google Scholar]
- 11.John R. A. (1993) in Enzyme Assays: A Practical Approach, eds. Eisenthal, R. & Danson, M. J. (Oxford Univ. Press, New York), pp. 76–78.
- 12.Miwa H. (2000) J. Chromatogr. 881, 365-385. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro C. S., Reis, M., Panizzutti, R., de Miranda, J. & Wolosker, H. (2002) Brain Res. 929, 202-209. [DOI] [PubMed] [Google Scholar]
- 14.Schwartzenbach G., Senn, H. & Anderegg, G. (1957) Helv. Chim. Acta 40, 1186-1194. [Google Scholar]
- 15.Wolosker H., Petretski, J. H. & De Meis, L. (1990) Eur. J. Biochem. 193, 873-877. [DOI] [PubMed] [Google Scholar]
- 16.Silver I. A., Deas, J. & Erecinska, M. (1997) Neuroscience 78, 589-601. [DOI] [PubMed] [Google Scholar]
- 17.Desagher S., Glowinski, J. & Premont, J. (1997) J. Neurosci. 17, 9060-9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheline C. T., Behrens, M. M. & Choi, D. W. (2000) J. Neurosci. 20, 3139-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J. Y., Kim, Y. H. & Koh, J. Y. (2001) J. Neurosci. 21, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soda K., Yoshimura, T. & Esaki, N. (2001) Chem. Rec. 1, 373-384. [DOI] [PubMed] [Google Scholar]
- 21.Wolosker H., D'Aniello, A. & Snyder, S. H. (2000) Neuroscience 100, 183-189. [DOI] [PubMed] [Google Scholar]
- 22.Brocard J. B., Rajdev, S. & Reynolds, I. J. (1993) Neuron 11, 751-757. [DOI] [PubMed] [Google Scholar]
- 23.Lo E. H., Pierce, A. R., Matsumoto, K., Kano, T., Evans, C. J. & Newcomb, R. (1998) Neuroscience 83, 449-458. [DOI] [PubMed] [Google Scholar]
- 24.Cook S. P., Galve-Roperh, I., Martinez Del Pozo, A. & Rodriguez-Crespo, I. (2002) J. Biol. Chem. 277, 27782-27792. [DOI] [PubMed] [Google Scholar]
- 25.Patil R. V. & Datta, P. (1988) Eur. J. Biochem. 177, 569-574. [DOI] [PubMed] [Google Scholar]