Abstract
Urinary excretion of orally administered lactulose and 51 chromium labelled ethylenediamine tetra-acetate (51Cr-EDTA) was measured in 12 healthy adult subjects and in six patients with ileostomies to assess intestinal permeability. In normal subjects, 24 hour urinary recovery of 51Cr-EDTA was significantly greater than that of lactulose (mean (SEM) 2.27 (0.15) v 0.50 (0.08)% oral dose; p less than 0.001), but in ileostomy patients recovery of the two markers was the same. In normal subjects, therefore, the difference between the two markers may arise from bacterial break-down of lactulose but not of 51Cr-EDTA in the distal bowel, urinary excretion of lactulose representing small intestinal permeation and that of 51Cr-EDTA representing both small and large intestinal permeation. The markers were then given simultaneously to nine patients receiving non-steroidal anti-inflammatory drugs (NSAIDs) for rheumatoid arthritis and osteoarthritis. The 24 hour urinary recovery of 51Cr-EDTA in the patients was significantly greater than normal (4.64 (1.20) v 2.27 (0.15)% oral dose; p less than 0.01), but that of lactulose was not significantly affected. Moreover, the increase in 51Cr-EDTA recovery was most noticeable in the later urine collections. Both of these findings suggest that NSAIDs may increase colonic permeability.
Full text
PDF
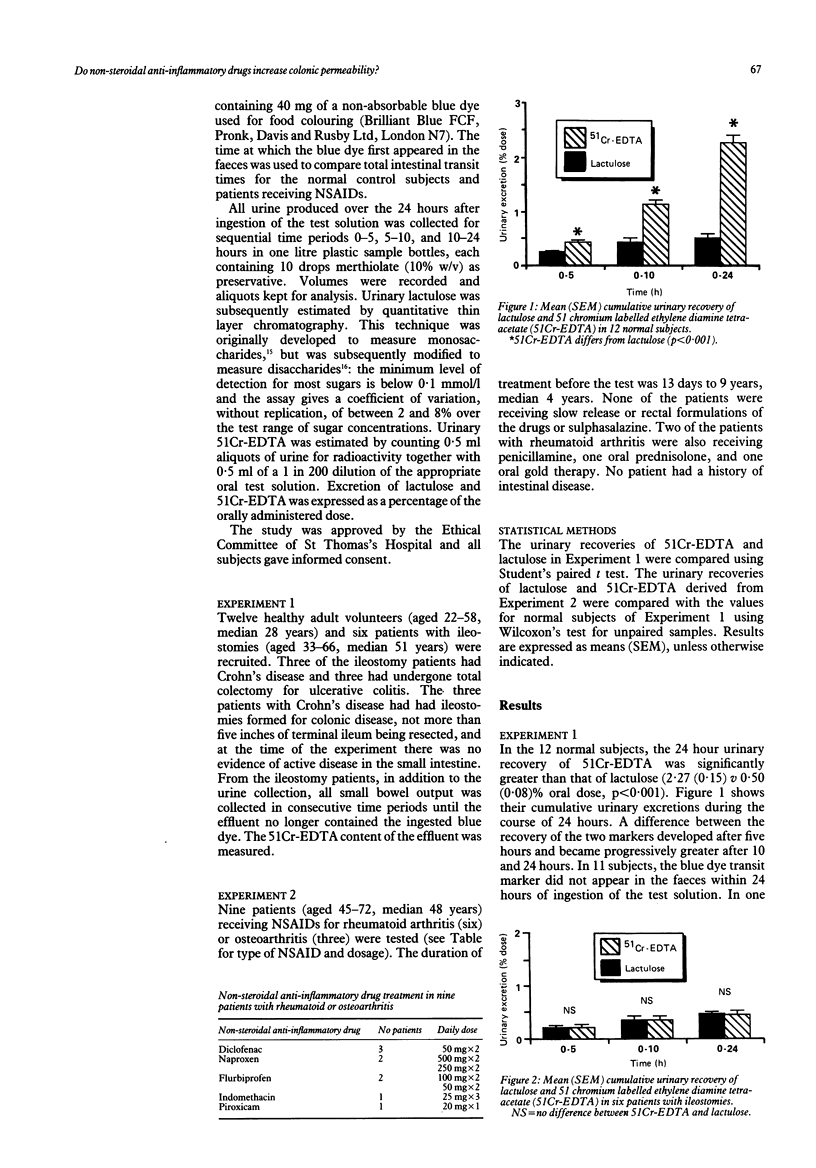
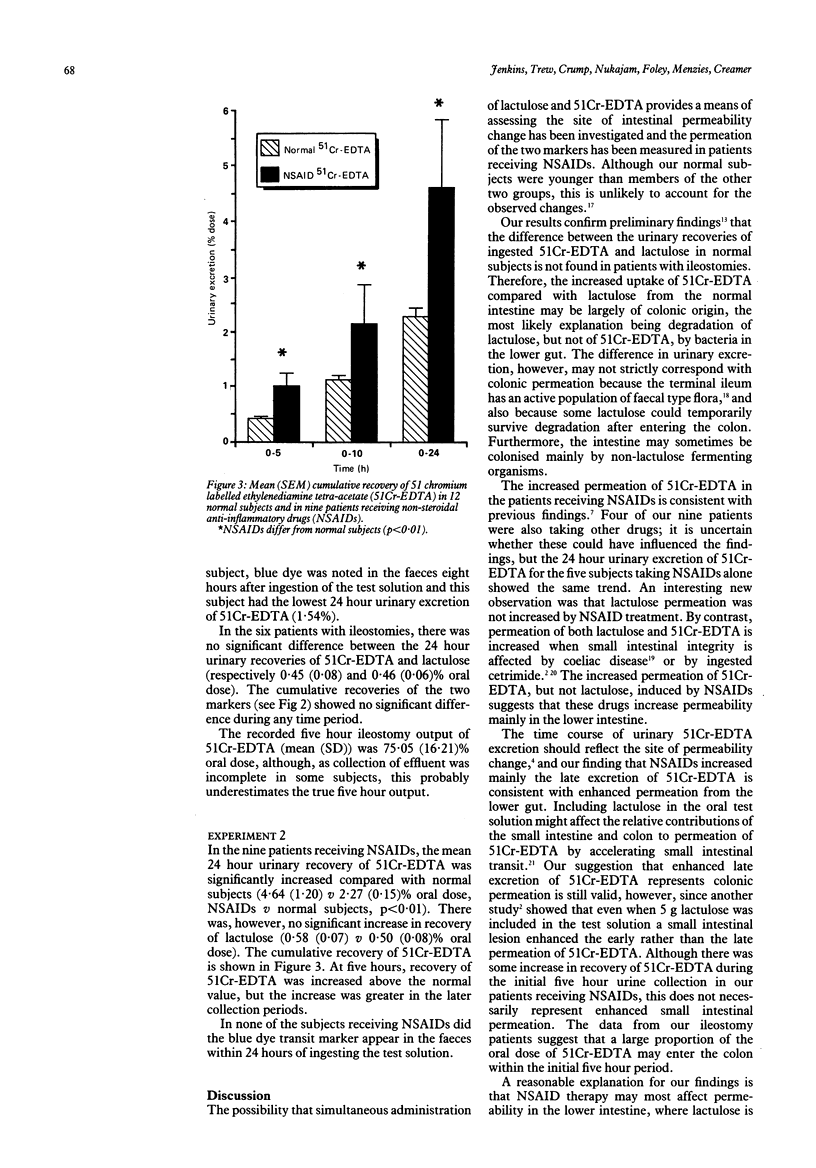

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjarnason I., O'Morain C., Levi A. J., Peters T. J. Absorption of 51chromium-labeled ethylenediaminetetraacetate in inflammatory bowel disease. Gastroenterology. 1983 Aug;85(2):318–322. [PubMed] [Google Scholar]
- Bjarnason I., Peters T. J., Veall N. A persistent defect in intestinal permeability in coeliac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet. 1983 Feb 12;1(8320):323–325. doi: 10.1016/s0140-6736(83)91628-8. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Williams P., So A., Zanelli G. D., Levi A. J., Gumpel J. M., Peters T. J., Ansell B. Intestinal permeability and inflammation in rheumatoid arthritis: effects of non-steroidal anti-inflammatory drugs. Lancet. 1984 Nov 24;2(8413):1171–1174. doi: 10.1016/s0140-6736(84)92739-9. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Prouse P., Williams P., Gumpel M. J., Levi A. J. Effect of non-steroidal anti-inflammatory drugs on the human small intestine. Drugs. 1986;32 (Suppl 1):35–41. doi: 10.2165/00003495-198600321-00007. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Black D. A. Intestinal permeability in the elderly. Dig Dis Sci. 1988 Mar;33(3):382–382. doi: 10.1007/BF01535768. [DOI] [PubMed] [Google Scholar]
- Borriello S. P. Bacteria and gastrointestinal secretion and motility. Scand J Gastroenterol Suppl. 1984;93:115–121. [PubMed] [Google Scholar]
- Elia M., Behrens R., Northrop C., Wraight P., Neale G. Evaluation of mannitol, lactulose and 51Cr-labelled ethylenediaminetetra-acetate as markers of intestinal permeability in man. Clin Sci (Lond) 1987 Aug;73(2):197–204. doi: 10.1042/cs0730197. [DOI] [PubMed] [Google Scholar]
- Holgate A. M., Read N. W. Relationship between small bowel transit time and absorption of a solid meal. Influence of metoclopramide, magnesium sulfate, and lactulose. Dig Dis Sci. 1983 Sep;28(9):812–819. doi: 10.1007/BF01296904. [DOI] [PubMed] [Google Scholar]
- Kaufmann H. J., Taubin H. L. Nonsteroidal anti-inflammatory drugs activate quiescent inflammatory bowel disease. Ann Intern Med. 1987 Oct;107(4):513–516. doi: 10.7326/0003-4819-107-4-513. [DOI] [PubMed] [Google Scholar]
- Langman M. J., Morgan L., Worrall A. Use of anti-inflammatory drugs by patients admitted with small or large bowel perforations and haemorrhage. Br Med J (Clin Res Ed) 1985 Feb 2;290(6465):347–349. doi: 10.1136/bmj.290.6465.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxton D. G., Bjarnason I., Reynolds A. P., Catt S. D., Peters T. J., Menzies I. S. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond) 1986 Jul;71(1):71–80. doi: 10.1042/cs0710071. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Laker M. F., Pounder R., Bull J., Heyer S., Wheeler P. G., Creamer B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979 Nov 24;2(8152):1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Mount J. N., Wheeler M. J. Quantitative estimation of clinically important monosaccharides in plasma by rapid thin layer chromatography. Ann Clin Biochem. 1978 Mar;15(2):65–76. doi: 10.1177/000456327801500116. [DOI] [PubMed] [Google Scholar]
- NISSIM J. A. Reduction of intestinal absorption by a synthetic chemical. Nature. 1960 Jan 23;185:222–224. doi: 10.1038/185222a0. [DOI] [PubMed] [Google Scholar]
- Noone C., Menzies I. S., Banatvala J. E., Scopes J. W. Intestinal permeability and lactose hydrolysis in human rotaviral gastroenteritis assessed simultaneously by non-invasive differential sugar permeation. Eur J Clin Invest. 1986 Jun;16(3):217–225. doi: 10.1111/j.1365-2362.1986.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Pearson A. D., Eastham E. J., Laker M. F., Craft A. W., Nelson R. Intestinal permeability in children with Crohn's disease and coeliac disease. Br Med J (Clin Res Ed) 1982 Jul 3;285(6334):20–21. doi: 10.1136/bmj.285.6334.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampton D. S., McNeil N. I., Sarner M. Analgesic ingestion and other factors preceding relapse in ulcerative colitis. Gut. 1983 Mar;24(3):187–189. doi: 10.1136/gut.24.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A., Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977 Aug;14(2):333–341. doi: 10.1016/0090-6980(77)90178-2. [DOI] [PubMed] [Google Scholar]
- Vogelsang H., Ferenci P., Frotz S., Meryn S., Gangl A. Acidic colonic microclimate--possible reason for false negative hydrogen breath tests. Gut. 1988 Jan;29(1):21–26. doi: 10.1136/gut.29.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]



