Abstract
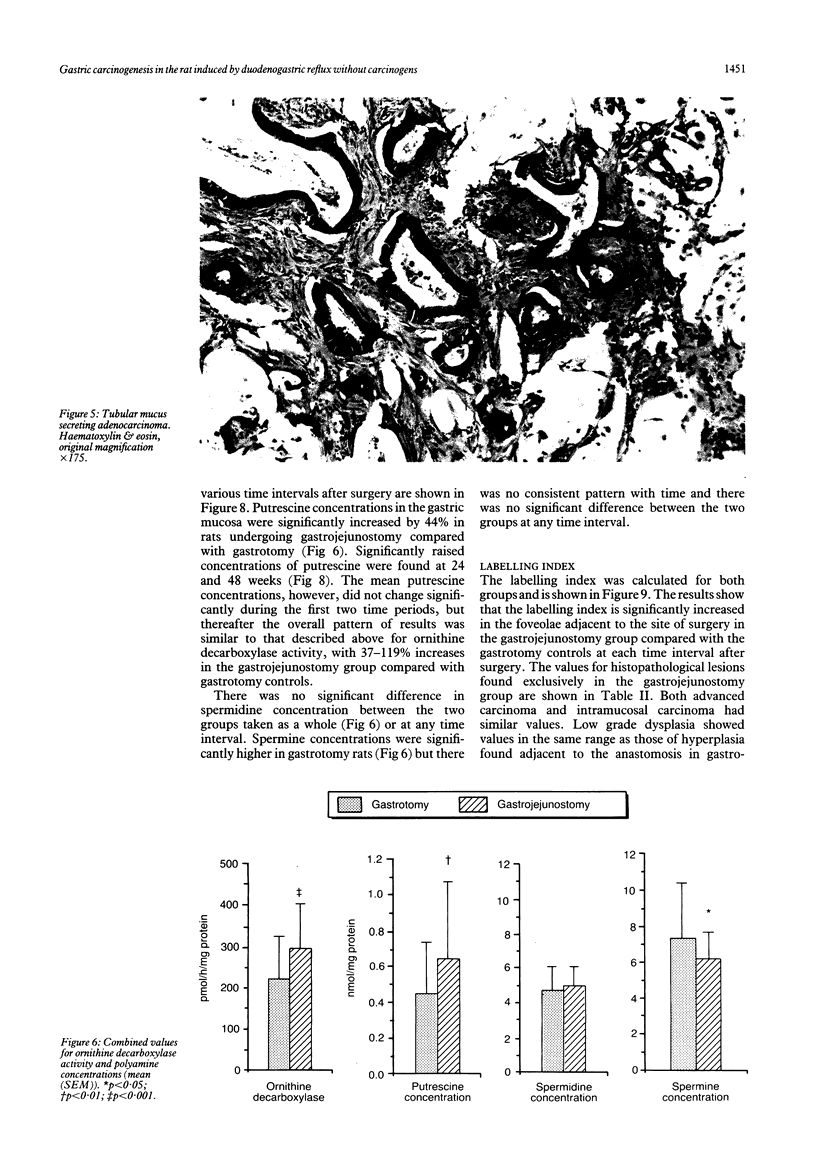
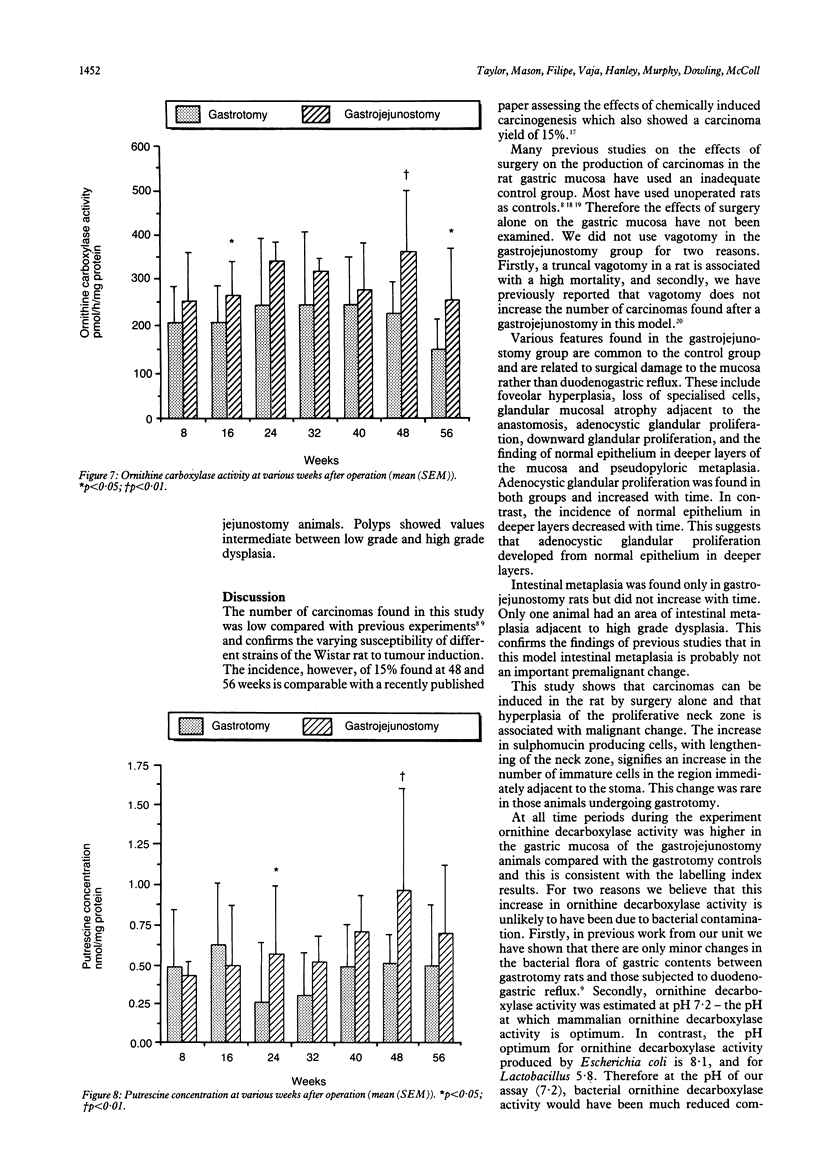
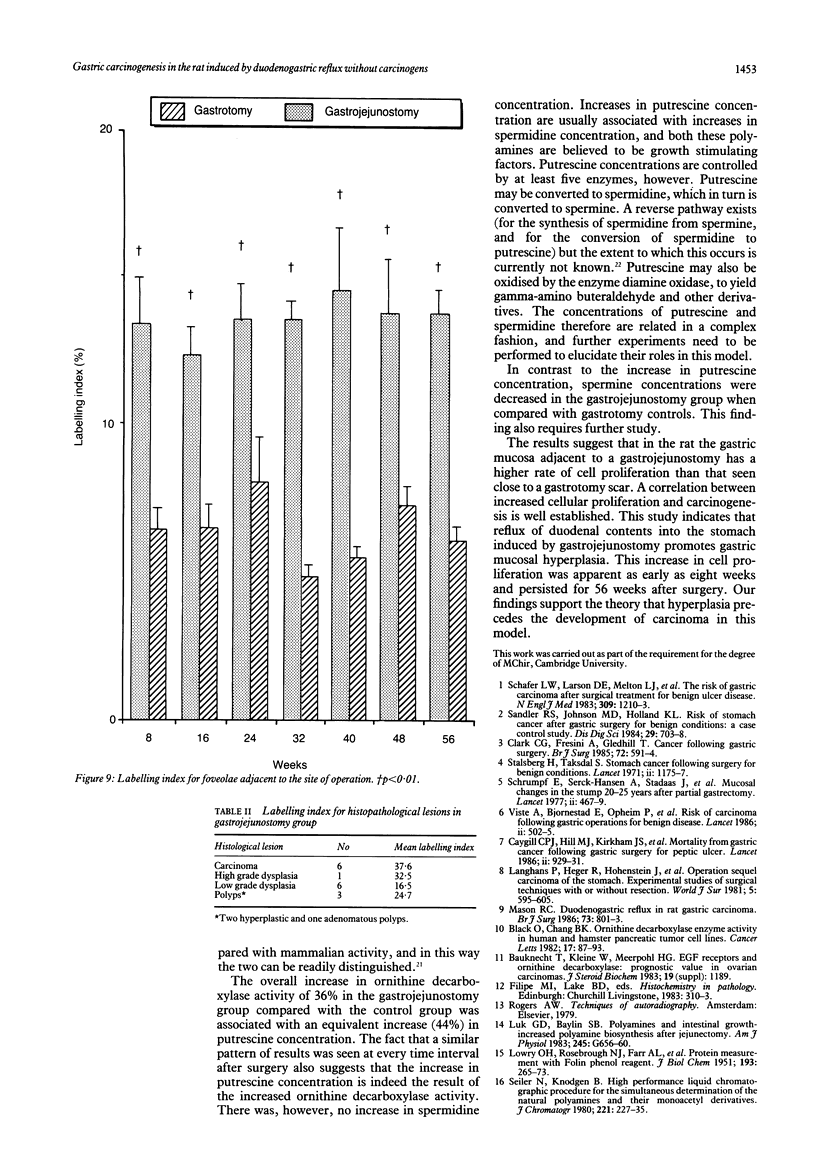
Chronic duodenogastric reflux induces gastric adenocarcinomas in the rat without the use of carcinogens. Altogether, 186 male Wistar rats were randomised to undergo either a simple gastrojejunostomy or a gastrotomy and sacrificed at eight weekly intervals for 56 weeks. No control animals developed dysplasia or carcinoma. All rats subjected to a gastrojejunostomy showed hyperplasia of the proliferative neck zone, with increased sulphomucin production adjacent to the scar. Low grade dysplasia was found at 16 weeks, and carcinoma was first seen at 32 weeks. Most carcinomas were well differentiated mucin secreting adenocarcinomas of the expanding type, which secreted a mixture of sialomucins and sulphomucins. Duodenogastric reflux was associated with a 100% increase in labelling index (assessed autoradiographically with tritiated thymidine) in the gastric mucosa when compared with corresponding tissue adjacent to a gastrotomy scar. This increase was significant at eight weeks and persisted for 56 weeks after surgery. This study supports the theory that, in this model, hyperplasia precedes the development of carcinoma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black O., Jr, Chang B. K. Ornithine decarboxylase enzyme activity in human and hamster pancreatic tumor cell lines. Cancer Lett. 1982 Oct;17(1):87–93. doi: 10.1016/0304-3835(82)90113-6. [DOI] [PubMed] [Google Scholar]
- Brooks C. J., Cole W. J., Lawrie T. D., MacLachlan J., Borthwick J. H., Barrett G. M. Selective reactions in the analytical characterisation of steroids by gas chromatography-mass spectrometry. J Steroid Biochem. 1983 Jul;19(1A):189–201. [PubMed] [Google Scholar]
- Caygill C. P., Hill M. J., Kirkham J. S., Northfield T. C. Mortality from gastric cancer following gastric surgery for peptic ulcer. Lancet. 1986 Apr 26;1(8487):929–931. doi: 10.1016/s0140-6736(86)91041-x. [DOI] [PubMed] [Google Scholar]
- Clark C. G., Fresini A., Gledhill T. Cancer following gastric surgery. Br J Surg. 1985 Aug;72(8):591–594. doi: 10.1002/bjs.1800720803. [DOI] [PubMed] [Google Scholar]
- Houghton P. W., Mortensen N. J., Williamson R. C. Effect of duodenogastric reflux on gastric mucosal proliferation after gastric surgery. Br J Surg. 1987 Apr;74(4):288–291. doi: 10.1002/bjs.1800740421. [DOI] [PubMed] [Google Scholar]
- Kaye A. M. Ornithine decarboxylase. Purification and properties of ornithine decarboxylase. Cell Biochem Funct. 1984 Jan;2(1):2–6. doi: 10.1002/cbf.290020102. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langhans P., Heger R. A., Hohenstein J., Schlake W., Bünte H. Operation-sequel carcinoma of the stomach. Experimental studies of surgical techniques with or without resection. World J Surg. 1981 Jul;5(4):595–605. doi: 10.1007/BF01655015. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Polyamines and intestinal growth--increased polyamine biosynthesis after jejunectomy. Am J Physiol. 1983 Nov;245(5 Pt 1):G656–G660. doi: 10.1152/ajpgi.1983.245.5.G656. [DOI] [PubMed] [Google Scholar]
- Mason R. C. Duodenogastric reflux in rat gastric carcinoma. Br J Surg. 1986 Oct;73(10):801–803. doi: 10.1002/bjs.1800731014. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler R. S., Johnson M. D., Holland K. L. Risk of stomach cancer after gastric surgery for benign conditions. A case-control study. Dig Dis Sci. 1984 Aug;29(8):703–708. doi: 10.1007/BF01312941. [DOI] [PubMed] [Google Scholar]
- Schafer L. W., Larson D. E., Melton L. J., 3rd, Higgins J. A., Ilstrup D. M. The risk of gastric carcinoma after surgical treatment for benign ulcer disease. A population-based study in Olmsted County, Minnesota. N Engl J Med. 1983 Nov 17;309(20):1210–1213. doi: 10.1056/NEJM198311173092003. [DOI] [PubMed] [Google Scholar]
- Schrumpf E., Serck-Hanssen A., Stadaas J., Aune S., Myren J., Osnes M. Mucosal changes in the gastric stump 20-25 years after partial gastrectomy. Lancet. 1977 Sep 3;2(8036):467–469. doi: 10.1016/s0140-6736(77)91599-9. [DOI] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980 Dec 12;221(2):227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- Stalsberg H., Taksdal S. Stomach cancer following gastric surgery for benign conditions. Lancet. 1971 Nov 27;2(7735):1175–1177. doi: 10.1016/s0140-6736(71)90489-2. [DOI] [PubMed] [Google Scholar]
- Viste A., Bjørnestad E., Opheim P., Skarstein A., Thunold J., Hartveit F., Eide G. E., Eide T. J., Søreide O. Risk of carcinoma following gastric operations for benign disease. A historical cohort study of 3470 patients. Lancet. 1986 Aug 30;2(8505):502–505. doi: 10.1016/s0140-6736(86)90368-5. [DOI] [PubMed] [Google Scholar]