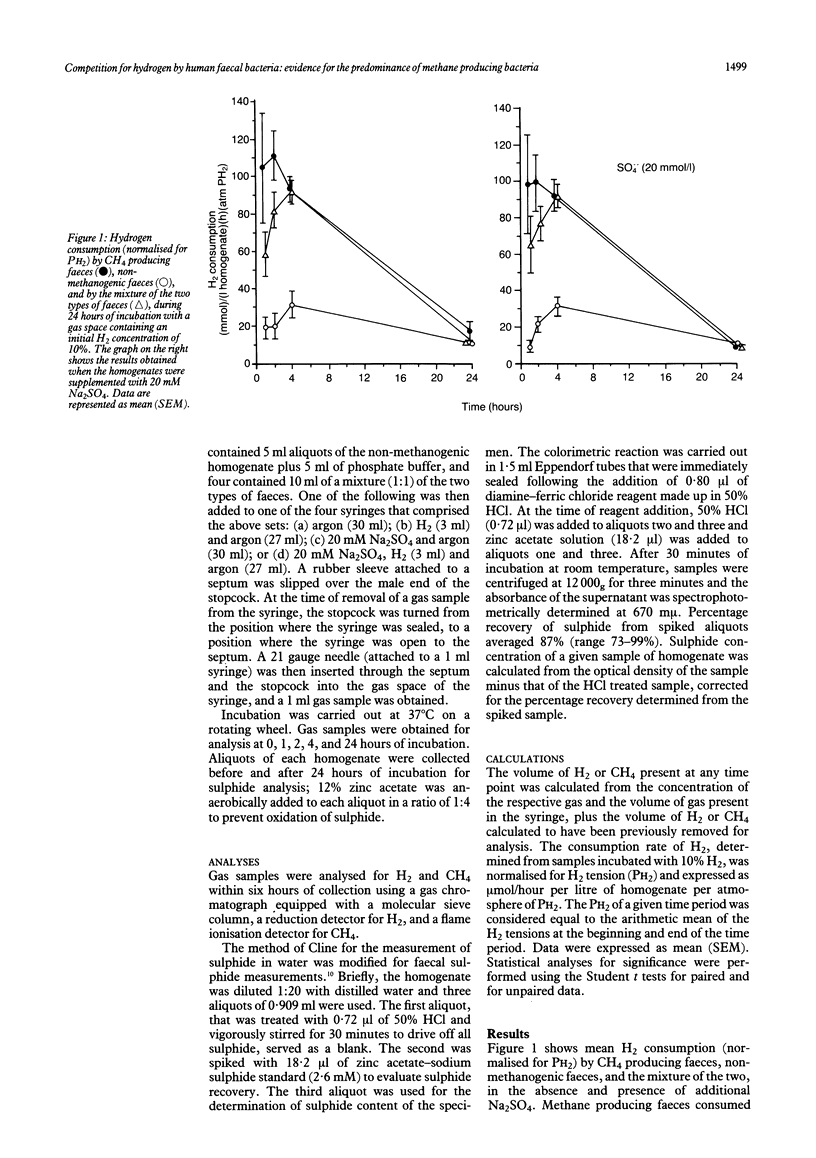
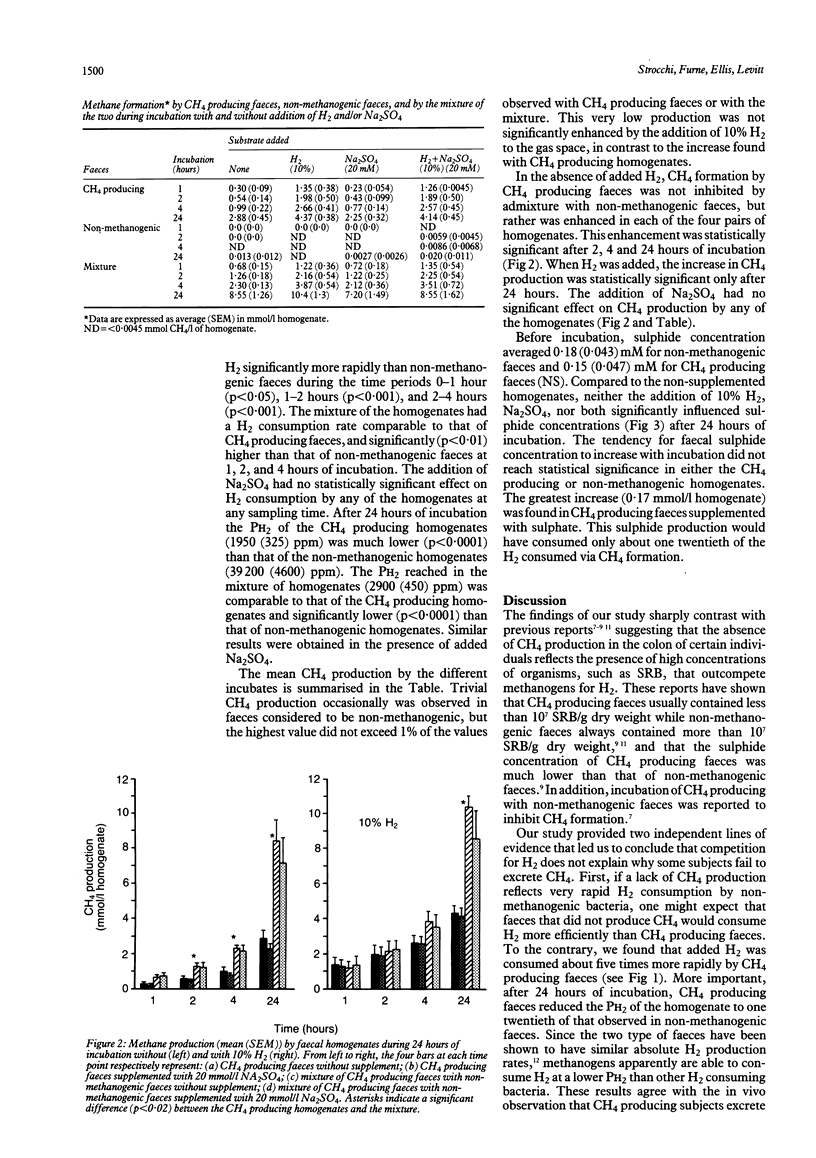
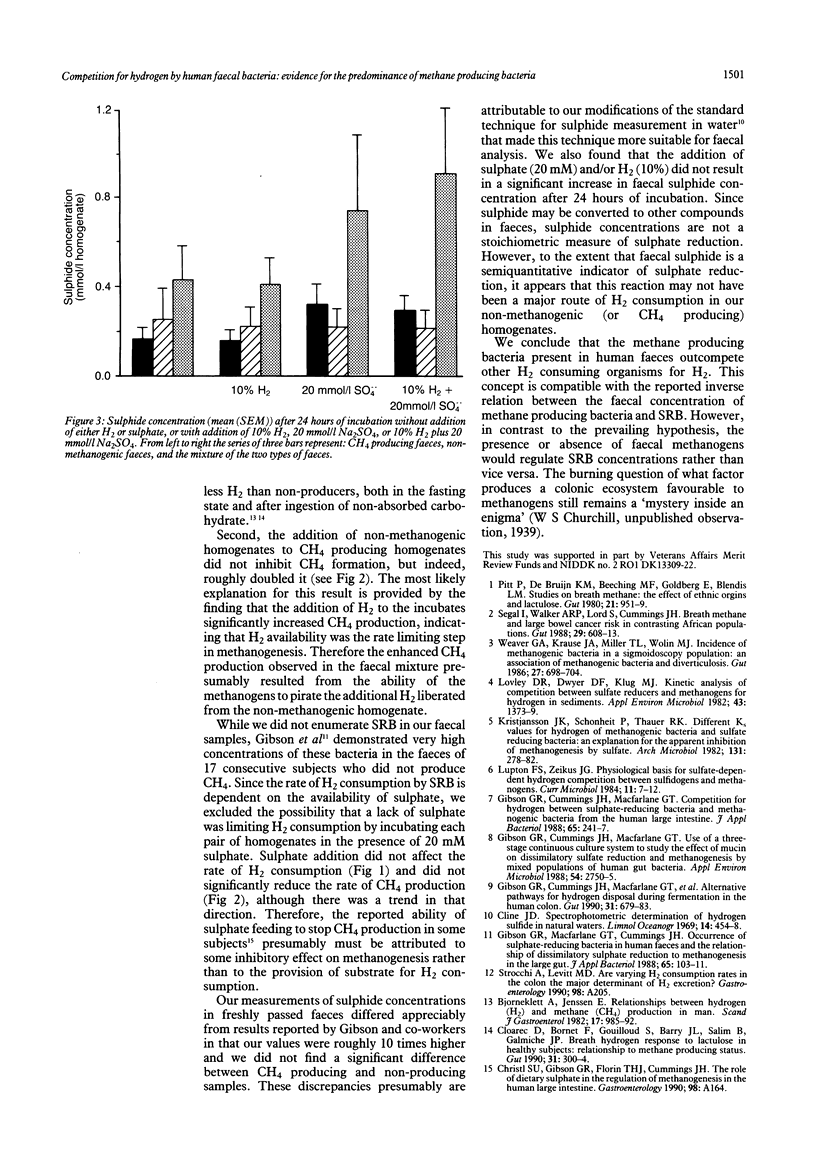
Abstract
Studies of sludge have shown that some species of sulphate reducing bacteria outcompete methane producing bacteria for the common substrate H2. A similar competition may exist in human faeces where the methane (CH4) producing status of an individual depends on the faecal concentration of sulphate reducing bacteria. To determine if non-methanogenic faeces outcompete CH4 producing faeces for H2, aliquots of each type of faeces were incubated alone or mixed together, with or without addition of 10% H2 and/or 20 mmol/l sulphate. Methane producing faeces consumed H2 significantly more rapidly and reduced faecal H2 tension to a lower value compared with non-methanogenic faeces. The mixture of the two types of faeces yielded significantly more CH4 than CH4 producing faeces alone (mean (SD) 8.5 (1.3) v 2.9 (0.45) mmol/l of homogenate per 24 hours, p less than 0.01). Faecal sulphide concentrations were similar in CH4 producing and non-producing homogenates both before and after 24 hours of incubation. The addition of sulphate to the homogenates did not significantly influence CH4 production or sulphide formation. Our results suggest that in human faeces methane producing bacteria outcompete other H2 consuming bacteria for H2.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjørneklett A., Jenssen E. Relationships between hydrogen (H2) and methane (CH4) production in man. Scand J Gastroenterol. 1982 Nov;17(8):985–992. [PubMed] [Google Scholar]
- Cloarec D., Bornet F., Gouilloud S., Barry J. L., Salim B., Galmiche J. P. Breath hydrogen response to lactulose in healthy subjects: relationship to methane producing status. Gut. 1990 Mar;31(3):300–304. doi: 10.1136/gut.31.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Cummings J. H., Macfarlane G. T., Allison C., Segal I., Vorster H. H., Walker A. R. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990 Jun;31(6):679–683. doi: 10.1136/gut.31.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Cummings J. H., Macfarlane G. T. Competition for hydrogen between sulphate-reducing bacteria and methanogenic bacteria from the human large intestine. J Appl Bacteriol. 1988 Sep;65(3):241–247. doi: 10.1111/j.1365-2672.1988.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Cummings J. H., Macfarlane G. T. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol. 1988 Nov;54(11):2750–2755. doi: 10.1128/aem.54.11.2750-2755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Macfarlane G. T., Cummings J. H. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol. 1988 Aug;65(2):103–111. doi: 10.1111/j.1365-2672.1988.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Dwyer D. F., Klug M. J. Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl Environ Microbiol. 1982 Jun;43(6):1373–1379. doi: 10.1128/aem.43.6.1373-1379.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt P., de Bruijn K. M., Beeching M. F., Goldberg E., Blendis L. M. Studies on breath methane: the effect of ethnic origins and lactulose. Gut. 1980 Nov;21(11):951–954. doi: 10.1136/gut.21.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal I., Walker A. R., Lord S., Cummings J. H. Breath methane and large bowel cancer risk in contrasting African populations. Gut. 1988 May;29(5):608–613. doi: 10.1136/gut.29.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver G. A., Krause J. A., Miller T. L., Wolin M. J. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986 Jun;27(6):698–704. doi: 10.1136/gut.27.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]


