Abstract
Psychological and neurobiological theories of cognitive control must account for flexible, seemless transitions among cognitive operations. When subjects switch between tasks, they must both inhibit the previous task and re-engage in a different task. Inhibition of the disengaged task remains active for a period of time and has to be overcome when re-engaging in the same task. Here we used a task-switching paradigm that allows distinction of two control processes: overcoming the inhibition of a previously performed task when re-engaging it and restarting a sequence of tasks after a period of interruption. Behaviorally, these processes were reflected in the facts that: (i) switching to a recently performed task, that is thus unlikely to have fully recovered from inhibition, takes longer than switching to a task less recently performed and (ii) re-engaging in a sequence of tasks after a period of interruption transiently increases response time. Using event-related functional MRI, we found that these two behavioral effects were accompanied by a double dissociation: the right lateral prefrontal cortex was more activated when switching to a task recently performed compared to a task less recently performed, while the anterior cingulate cortex was recruited when a sequence of tasks was initiated. These results provide insights into the functional organization of the frontal lobe in humans and its role in distinct processes involved in cognitive control.
Keywords: prefrontal, cingulate, executive processes, task switching, inhibition
Cognitive control is the ability to flexibly adapt behavior to current demands by promoting task-relevant information in the face of interference or competition. The different processes involved in cognitive control and their corresponding neural substrates remain poorly identified because control processes are an integral part of the performance of every task, making it difficult to isolate their contribution from those of direct processing and response activities. However, task-switching paradigms requiring subjects to perform sequences of decisions based on cognitive rules have the potential to elucidate and disentangle subcomponents of control processes. Recent behavioral studies of task switching have reported that at least two distinct control processes can be distinguished: (i) the re-engagement in a task after an interruption and (ii) the disengagement from a previously performed task (1–4).
When subjects re-engage in a sequence of tasks following an interruption, there is an increased response time, or “restart cost,” which is present whether or not the re-engaged task is the same as the last task performed before the interruption (3, 4). This interruption can either be a simple rest period (3) or an instruction cue to either switch between tasks or continue the same task (4). The restart cost is likely to reflect a general reorienting or alertness effect triggered by the occurrence of the stimulus initiating a sequence of tasks. The nature of the restart cost is at least in part automatic because it is seen even when subjects know in advance that there will be no change between two separated blocks of a single task (4). The restart cost observed when re-engaging in a task has been proposed to be related to the “switch cost,” i.e., the increase in response time typically observed on the first trial of a run when subjects switch between two tasks as compared to simply repeating the same task (5–7). Identification of the neural basis of the restart cost is, thus, important to better understand the basic brain mechanisms by which we constantly adapt behavior to current and changing demands.
A second cognitive control component involved in the seamless transition between different cognitive demands, that has been behaviorally examined in task-switching studies, is the disengagement from the task just performed, referred to as “backward inhibition” (1, 2, 8). This inhibition of the disengaged task remains active for a period and must be overcome when it again becomes necessary to re-engage in the same, original task. The presence of backward inhibition is demonstrated behaviorally by the fact that switching to a task that has recently been performed, and is, thus, unlikely to have fully recovered from inhibition, takes longer than switching to a task less recently performed (1, 2). Backward inhibition of a no-longer relevant task has been proposed to be automatically triggered by competition between cognitive demands during task disengagement because it occurs even when subjects know that the inhibited task will become relevant again in the immediate future (1). The backward inhibition effect is an important result because inhibition has been proposed to be a component process of executive control, but it has been difficult to establish empirically (8, 9).
As the process of overcoming the residual inhibition of a recently performed task concerns the effect of previous trials' performance on current ones, it is instructive to examine previous neuroimaging studies that have examined the neural basis of similar effect. It has been shown that inhibitory functions related to previous trials involve the lateral prefrontal cortex (PFC) during a working memory task (10–12). In these studies, subjects were presented with a set of target letters for storage followed, after a delay period, by a probe letter, and they indicated by pressing a left- or right-hand button whether or not the probe was a member of the target set. Subjects were slower to respond if the probe was highly recent (in the previous target letter set) than they were if it was less recent (not in either of the two previous target letter sets). This effect may reflect at least two types of inhibitory processes: (i) inhibition at the cognitive level, i.e., inhibition of the target stimulus that had to be actively memorized in a previous trial and that subsequently needed to be inhibited so as not to interfere with the current trial; and (ii) inhibition at the motor level, i.e., when a probe did not match a target and, therefore, required a “no” response, the probe had matched a target of the previous trial, so on these trials a “yes” response was prepotent and had to be inhibited (10). These studies did not distinguish between these two processes. Moreover, they leave open the question of whether the observed lateral PFC activation may be due to the requirement, intrinsic to this paradigm, of actively maintaining previous targets in working memory, which in itself requires similar lateral prefrontal regions (13, 14). This possibility is supported by theoretical and experimental accounts supporting a strong link between working memory and inhibitory processes (12, 15, 16). In particular, the efficiency with which information is maintained in working memory is closely related to the ability to dispense with older information (17).
Thus, it is unknown whether the lateral PFC is also needed to overcome the influence of a previous task on the actual performance when subjects perform one task and then another without the need to remember information during a delay period. In other words, it is unknown whether overcoming residual inhibition during task switching, per se, involves the lateral PFC. The goal of the current event-related functional MRI (fMRI) study was to investigate the neural basis of these two component processes (overcoming the residual inhibition of a recent task and re-engagement in a task after a period of interruption) involved in sequences of cognitive activities.
We hypothesized that overcoming inhibition of a previously performed task would involve the lateral PFC during task switching, as is the case in the working memory studies mentioned above (10–12). We also predicted that the anterior cingulate (ACC) would be activated with re-engagement in a task sequence, as suggested by patients with ACC lesions that often show deficits in spontaneous initiation of movements or speech (18, 19). The role of the medial wall in task re-engagement can also be anticipated because neurons from the pre-supplementary motor area (pre-SMA) have been shown to be specifically active for the first movement of a renewed motor sequence guided by visual stimuli (20).
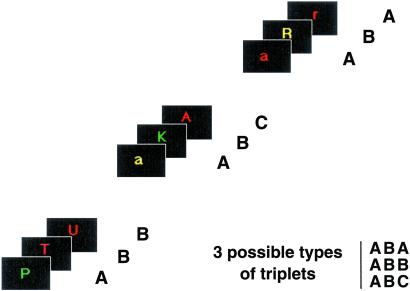
To study brain regions needed to overcome inhibition of a previous cognitive task and those necessary to re-engage in the first task of a cognitively based sequence after a period of interruption, we developed for event-related fMRI a task-switching paradigm requiring three types of letter discrimination tasks (vowel/consonant, lower/uppercase, and before/after m in the alphabet), each of which was specifically cued by the color of the letter (red, green, and yellow, respectively). Single letters were successively presented in triplets separated by a variable rest period. Unbeknowst to the subjects, triplets of tasks were constructed to vary task repetition, switching, and recency within the three-letter sets (ABA, ABB, and ABC, where A, B, and C reflect task order within a triplet, but can indicate any of the three tasks) (Fig. 1). For example, ABA represents the triplets RGR, RYR, GRG, GYG, YRY, and YGY (where R, G, and Y designate red, green, and yellow letters, respectively). This paradigm surmounts limitations of previous fMRI studies of task switching that have used only two tasks in alternation and could not specifically examine overcoming the residual inhibition of a recently performed task because the new task to be performed was also the one most recently disengaged (21–23).
Fig 1.
Experimental paradigm. Subjects responded to single color letters by pressing response buttons held in each hand. Letters were successively presented every 2.5 s (duration 1.5 s) in triplets separated by 5 or 9 s. Triplets were constructed to vary task repetition, switching, and recency within the three-letter sets (ABA, ABB, and ABC, where A, B, and C reflect task order within a triplet, but can indicate any of the three tasks). Indicated are three particular examples of the triplets ABB, ABC, and ABA.
We tested whether overcoming inhibition of a previously performed task involved the lateral PFC by comparing neural activity associated with switching to a task recently performed to that when switching to a less recent task (contrast ABA>ABC for the third element of a triplet). Our paradigm avoids working memory confounds and controls for the effect of previous motor responses on current ones, thus, allowing investigation of brain regions specifically involved in overcoming cognitive, rather than motor, inhibition. Furthermore, we tested whether the ACC would be more activated with re-engagement in a sequence of tasks after a period of interruption by contrasting activation in the first task of a triplet relative to the other two.
Methods
Behavioral Protocol.
Fourteen right-handed subjects (seven males; mean age = 28.6, range 22–35) were recruited following the procedures approved by the National Institute of Mental Health Institutional Review Board. One day before the MR session, subjects participated in a behavioral testing session during which they were trained to perform the tasks. Subjects responded to single color letters by pressing response buttons held in each hand (Fig. 1). There were three tasks, each specifically cued by the color of the letter. If the letter was red, subjects indicated whether it was a vowel (right button) or a consonant (left button). If the letter was green, subjects had to discriminate whether it was uppercase (right) or lowercase (left). Finally, if the letter was yellow, subjects indicated whether it was before m in the alphabet (right) or after m (left). Single color letters were successively presented every 2.5 s (duration 1.5 s) in triplets separated by an inter-trial interval of 5 s (probability = 1/3) or 9 s (probability = 2/3). Triplets were constructed to vary task repetition, switching, and recency within the three-letter sets (ABA, ABB, and ABC, where A, B, and C reflect task order within a triplet). The tasks were administered in six scanning runs of 72 letters (eight triplets of each of the three types ABA, ABB, and ABC) by using EXPE 6 software (www.ehess.fr). In each run, there was an equal number of each task and an equal number of left and right motor responses.
Image Acquisition and Analysis.
A 1.5-T GE Signa scanner equipped with a radio frequency coil was used to acquire both T1 anatomical volume images (1.2 mm thick, 0.9 mm cubic voxels) and T2*-weighted spiral images [repetition time (TR) = 2 s, echo time (TE) = 24 ms, flip angle = 85, 64*64 matrix, 3.75 mm cubic voxels] with blood oxygenation level-dependent contrast acquired axially in six runs. Each run was comprised of 162 volumes of 36 slices covering the whole brain. The first four volumes were discarded to allow for T1 equilibration. Using SPM 99 (Statistical Parametric Mapping) software, volumes were realigned by using sinc interpolation, slice timing corrected, registered and normalized to Talairach coordinates via a linear transform calculated on the anatomical images. The normalized functional images were smoothed by using a 10-mm full-width half-maximum (FWHM) isotropic Gaussian kernel and globally scaled to 100.
Statistical analysis was performed by using the general linear model in SPM 99, using a random effect model. The responses to stimulus onsets for each event type, synchronized with the acquisition of the middle slice, were modeled by a canonical hemodynamic response function. We performed a random effect analysis to investigate brain regions involved with the two main contrasts (contrasts ABA>ABC and first task>(second + third tasks)/2). We specified six effects of interest: first task (switch or repeat), second task, third task (ABA, ABB, ABC). The random-effect analysis involved three steps. Session-specific parameter estimates pertaining to the hemodynamic response function for each effect of interest were calculated for each voxel. Then, an appropriate contrast of parameter estimates across sessions was calculated in a voxel-wise manner to produce, for each subject, one contrast for each experimental condition. Finally, for each comparison of interest, the appropriate contrast images from each individual were entered into a one-sample t test performed across all subjects.
Given our a priori hypotheses concerning the lateral PFC and ACC activation, we used a significance threshold of P < 0.001, uncorrected, with a cluster level of P < 0.05, corrected for multiple comparisons (corresponding to 21 contiguous voxels).
Results
Behavioral Data.
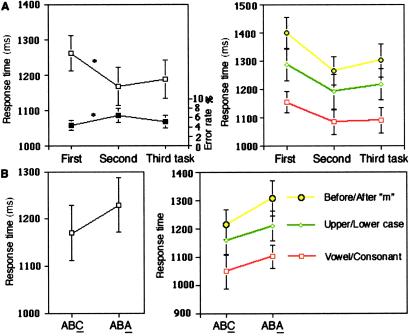
Behavioral results were first analyzed by using repeated measures ANOVA with position in a triplet (first, second, and third) and type of task (vowel/consonant, upper/lowercase, and before/after m) as within-subject factors. Response times (RTs) were examined for correct trials only (RTs <2,400 ms). Regarding RTs, as expected, there was a main effect of position in the triplet [F(2, 26) = 20.3, P < 0.0001], consistent with a restart cost: the first task of a triplet induced slower RTs than the second task [F(1, 13) = 25.0, P < 0.001] or the third task [F(1, 13) = 28.4, P < 0.0005] (Fig. 2A Left). There was also a main effect of task type [F(2, 26) = 26.1, P < 0.0001]: RTs in the before/after m task were slower than those of the upper/lowercase task [F(1, 13) = 15.3, P < 0.01], which were, themselves, slower than RTs of the vowel/consonant task [F(1, 13) = 15.9, P < 0.01]. Furthermore, the restart cost did not depend on task type; there was no RT interaction between position in a triplet and the type of task [F(4, 52) = 1.23, P = 0.31], indicating that the restart cost is not specific to a particular task (Fig. 2A Right).
Fig 2.
Behavioral results. (A Left) Effect of task position in a triplet of tasks. The first task was significantly slower than the second and third tasks (RT: □). This restart cost is a measure of the re-engagement process specifically involved in the first task and was associated with a reduction of error rates (error rates: ▪). (Right) The re-engagement effect was present for all tasks (no RTs interaction was present between the restart cost and the type of task). (B Left) Effect of overcoming the residual inhibition of a recently performed task. Switching to a recently performed task (the A in an ABA triplet), likely to suffer from residual inhibition, increased RT as compared to switching to a task that was performed less recently (the C in an ABC triplet). (Right) No RTs interaction was found between overcoming the residual inhibition of a recent task and task type.
Subjects made few errors (average error rates <7%). There was a significant error rate difference between positions in the triplet [F(2, 26) = 6.5, P < 0.01], the error rate in the first task being reduced as compared to the second task [F(1, 13) = 12.7, P < 0.005] (Fig. 2A Left). No significant difference was observed for the error rates when comparing the first task to the third [F(1, 13) = 1.6, P = 0.23], but the second task significantly differed from the third [F(1, 13) = 4.8, P < 0.05]. There was also a main effect of task type for the error rates [F(2, 26) = 28.5, P < 0.0001]. That is, the vowel/consonant task induced less errors than the upper/lowercase discrimination task [F(1, 13) = 39.3, P < 0.0001] or the before/after m task [F(1, 13) = 28.6, P < 0.0001]. Finally, there was an interaction between the position in a triplet and the task type for the error rates [F(4, 52) = 5.8, P < 0.001]. This interaction was because more errors were present for the second position in a triplet in the upper/lowercase discrimination task.
Second, we examined whether our paradigm confirms the behavioral backward inhibition effect previously observed with other tasks (1, 2). We, thus, concentrated on the third task of the triplet ABA and ABC, by performing a repeated measures ANOVA that included type of triplet for the third task (ABA, ABC) and type of task as factors. This analysis confirmed the backward inhibition effect: switching to a recently performed task, (the A in an ABA triplet), which is likely to still suffer from residual inhibition, increased RTs as compared to switching to a task that was performed less recently (the C in an ABC triplet) [F(1, 13) = 11.3, P < 0.01] (Fig. 2B Left). There was no significant difference in this comparison for error rates [F(1, 13) = 0.0, P = 0.95]. There was a main effect of task type for the third event [F(2, 26) = 22.4, P < 0.0001]: RTs in the before/after m task were slower than those of the upper/lowercase task, which were themselves, slower than RTs of the vowel/consonant task. This main effect of task type was also observed when averaging all three positions across a triplet [F(2, 26) = 26.3, P < 0.0001]. Importantly, backward inhibition did not depend on task type; there was no RT interaction between the triplet type (ABA vs. ABC) and the type of task [F(2, 26) = 0.9, P = 0.41] (Fig. 2B Right), indicating that the inhibition was not specific to a particular task.
Finally, to ensure that overcoming residual inhibition of a recent task occurred only in the cognitive and not in the motor domain, we also tested for possible interactions between positive motor priming (i.e., reduced RTs with repetition of the same hand of response) and the RT increase with switching to a recently performed task. We thus performed a repeated measures ANOVA, which included the type of triplet (ABA, ABC) for the third task and the repetition of the motor response (same or different hand) as factors. This latter analysis first compared the first and third tasks of a triplet and then the second and third tasks. If overcoming residual inhibition of a recently performed task occurred at a motor level, we would expect RT interactions between the increase with task switch recency and repetition of the same motor response between the first and third tasks or between the second and third tasks. However, there were no such interactions when the first and third tasks [F(1, 13) = 0.1, P = 0.76] or the second and third tasks [F(1, 13) = 1.1, P = 0.32] were considered.
Brain Imaging Data.
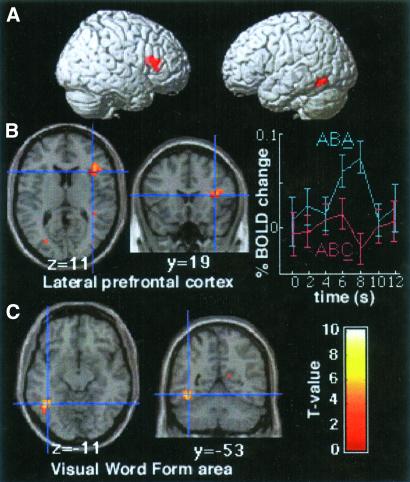
In the contrast designed to identify neural circuits related to overcoming residual inhibition (ABA > ABC, Fig. 3), increased neural activity was observed within the right lateral PFC when switching to a recently performed task was compared to switching to a less recently disengaged task. Two contiguous peaks of activation were observed [Brodmann area (BA) 45, x,y,z = 38,19,11, Z-value (Z) = 3.9 and BA 9/46, x,y,z = 27,27,27, Z = 4.2] that delineated an activated region primarily situated in the right ventro-lateral PFC with a smaller extension in the dorsolateral PFC. This finding is consistent with our hypothesis of the role of the lateral PFC in overcoming cognitive inhibition. Activation was also found in the left inferior temporal cortex (BA 37, x,y,z = −42,−53,−11; Z = 5.3) and the occipital cortex (BA 19, x,y,z = −27,−72,27; Z = 3.5).
Fig 3.
(A) Brain regions involved in overcoming residual inhibition of a recently performed task were overlaid onto a 3D rendered brain (contrast ABA > ABC for the third task). Activation was found in the right lateral PFC, the visual word form area, and the occipital cortex. (B Left) Location of the right lateral prefrontal region (BA 45; Talairach coordinates x,y,z = 38,19,11, Z = 3.9 and BA 9/46, x,y,z = 27,27,27, Z = 4.2) displaying greater activity when subjects switch to a task that has recently been performed as compared to switching to a task less recently performed. (Right) Averaged time series data for the group at the peak of the right lateral PFC for the ABA and ABC trials. (C) Location of the visual word form area (x,y,z = −42,−53,−11, Z = 5.3) displaying greater activity in the contrast ABA > ABC.
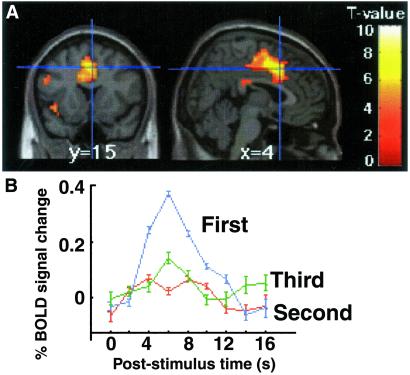
In the contrast examining the brain regions involved in the restart cost, the ACC was more activated for the first task of a triplet relative to the second and third tasks averaged together (x,y,z = 4,15,42, Z = 4.8) (Fig. 4). This ACC activation was specific to the first task because it was also found when comparing the first task to the second and the first task to the third, but there was no difference in ACC activity between the second task and the third (t = 0.6, Z = 0.7, P = 0.65, uncorrected). Furthermore, the ACC activation was found in the first task compared to the second and third tasks averaged together even if the re-engaged task was the same as the last task of the previous triplet (x,y,z = 4,8,42, Z = 5.1). The contrast comparing the first task of a triplet relative to the second and third tasks averaged together also identified the fusiform gyrus (BA 19, x,y,z = −42,−72,−8, Z = 3.9; x,y,z = 38,−65,−15, Z = 4.3), as well as bilateral premotor (BA 6, x,y,z = −49,4,30, Z = 4.8; x,y,z = 42,4,23, Z = 4.0) and motor cortices (x,y,z = −34,−11,49, Z = 5.2; x,y,z = 30,−27,49, Z = 4.5).
Fig 4.
(A) Location of the ACC region (BA 32, x,y,z = 4,15,42, Z = 4.8) displaying greater activity for the first task of a triplet relative to the second and third tasks averaged together. (B) Trial averaged time series data for the group at the peak of ACC cortex activation for the first, second, and third tasks of a triplet. Errors bars denote the SEM across participants.
To test whether there was effectively a double dissociation between the right lateral PFC and the ACC, we performed an additional region of interest (ROI) analysis. First, for each subject, we determined the t value of the two contrasts (ABA > ABC for the third task and first task>second task) in two spherical ROIs (radius = 5 mm) centered at the peak of maximal activity in the group analysis in the right lateral PFC (x,y,z = 27,27,27) and the ACC (x,y,z = 4,15,42). The resultant t values indicate, for each subject, the extent to which these two ROIs responded when overcoming cognitive inhibition and re-engaging in a sequence of tasks. Then, we performed a two-factor repeated measures ANOVA including brain regions (ACC vs. lateral PFC) and contrast (ABA > ABC vs. first task > second task) to assess whether the contrast effect differed across ROIs. There was a significant interaction between these two effects [F(1, 13) = 19.9, P < 0.001], demonstrating the double dissociation between these two brain regions.
Discussion
The present study allowed us to distinguish two component processes involved in cognitive control in general and task switching in particular and to dissociate their neural basis. The right lateral PFC was involved in overcoming the residual inhibition of a previously performed task while the ACC was selectively activated when re-engaging in the first task of a sequence of tasks, and there was a double dissociation between the two. Thus, these two brain regions appear to serve distinct and complementary processes during task switching, previously not distinguished (21–23). This is important because pinpointing the respective functions of these two frontal regions has proven difficult in neuroimaging studies, where they are often coactivated by control-demanding tasks (19, 24).
Lateral PFC Activation in Overcoming Cognitive Inhibition.
The behavioral effect found when overcoming residual inhibition supports theories of cognition arguing for the existence of inhibitory control (9, 25) and is therefore inconsistent with competing theories of cognitive control wherein activation alone is considered sufficient to explain selection between competing representations (15, 26). These latter theories would predict that a passive decay of activation engedered by the first task, A, should have facilitated RT for the repetition of this same task A in the ABA triplet, rather than the increased RT that we observed. Backward inhibition has been proposed to be automatically triggered by task competition during task disengagement and to last for a period of time. Thus, both the way it exerts control and the manner in which it is controlled are characteristic of a “low-level” process. This automatic nature of backward inhibition is consistent with the notion of inhibition as a general component of sequential control (1, 25). It should be noted that the lateral PFC activation that we found with overcoming residual inhibition was not specific to any of the three tasks in particular (e.g., the most difficult) because this effect was present for all tasks (Fig. 2B). The lateral PFC activity observed in our study does not reflect inhibition per se, but rather a response to the consequences of inhibition, specifically, overcoming residual inhibition from a recently performed task. This increased activity in lateral PFC seems to represent a heightened involvement of this region in situations where the appropriate task-set to be engaged needs more top-down support to win the competition with other task-sets.
As noted in the Introduction, the only previous brain imaging studies exploring the effect of previous trials on actual performance have used a working memory paradigm and could not demonstrate whether the lateral PFC activation found with inhibitory mechanism related to previous trials was independent of the requirement to actively maintain the targets in working memory (10–12). Our study shows that inhibition, although tightly related to working memory processes in some situations (12, 27), may also occur independently of these latter processes. Furthermore, it was unclear whether the behavioral effect reported in those studies reflected inhibition at the motor or the cognitive level, or yet other processes. In contrast, in our study, overcoming the residual inhibition of a recently performed task did not depend on previous motor responses because we found no interaction between this effect and motor priming. Thus, we believe our fMRI study adds an important piece to the puzzle by clearly demonstrating the role of the lateral PFC in overcoming inhibition at the cognitive level rather than at the motor level, and independently of the need to maintain information in working memory.
Alternative interpretations of our lateral PFC activation, such as selection among candidate memoranda (28), retrieval of the task-set (29), or maintenance of information in working memory are unlikely because these processes are all equally present for the third event in both the ABA and ABC conditions, and are therefore controlled for and subtracted in the comparison. This is important because inhibition has previously been difficult to distinguish from the above processes (8, 9).
Additional Brain Regions Involved in Overcoming Residual Inhibition.
Overcoming inhibition of a recently performed task (contrast ABA > ABC) also activated the occipital cortex (BA 19) and the left inferior temporal region (BA 37) (Fig. 3). Comparison with the coordinates identified in previously published work (30) shows that this left inferior temporal activation coincides with the “visual word form area” that is activated with character strings. Activation of these brain regions may reflect overcoming inhibition at the perceptual level (i.e., of the color of the letter of the first task of a triplet) in a manner analogous to the cognitive effect observed in the lateral PFC. That is, if the color associated with the first task is inhibited after performance of this task, inhibition of this color can carry over to a later trial and make it harder to perform the same task on a latter trial. Color information is known to be process at different stages. According to a classical scheme, the first stage of analysis occurs in V1 and V2 where simple wavelength information is registered, V4 occupies the second stage and is concerned with color constancy, and the final stage centers on the inferior temporal cortex that associates color with form (31). The visual word form area is, thus, at the ideal position to respond to the color of letters associated with a particular task.
ACC Activation in Re-Engagement in Task Sequence.
In contrast to the lateral PFC, the ACC was activated when initiating a sequence of tasks, suggesting that it implements a transient form of control in our study (Fig. 4). This ACC activation is consistent with observations that patients with ACC lesions often show deficits in spontaneous initiation of movements or speech (apathy, akinetic mutism) (18, 19). The transient ACC activation that we found when initiating a sequence of tasks has in common with the ACC activation previously found in error processing (32) that each may be considered as a transient form of control. However, our data indicate that an increase in alertness, or orienting of attention, in the first task may lead to decreased probability of errors (Fig. 2A) and that errors are not necessary to induce such transient ACC activity.
Recent studies have suggested a distinct functional relationship between the ACC and the lateral PFC (33–35). One current theory proposed that the ACC monitors the demand for cognitive control by detecting conflict situations and communicates with the lateral PFC, implementing the control when the need is detected (33). Other theories propose that the ACC is directly involved in the top-down control of attention (36), mediates an alerting/motivational function (34), or is involved in reward-based decision making (37). The peak of our ACC activation (x,y,z = 4,15,42) corresponds closely to those reported previously in evaluative process when detecting conflict situations, i.e., interference between different information-processing pathways [x,y,z = 0,15,41 (38) and x,y,z = 4,1,43 (33)]. Here we show that the evaluative process is not the only function of the ACC, because there was no more conflict for the first task than the other two. Thus, in the same way that ACC activation is not specific to error detection but is more generally involved in conflict situations (39), it appears that this ACC region is involved not only in conflict situations but in a more general alertness, or orienting, function.
The alertness function of the ACC is consistent with anatomy and neurophysiology because the ACC receives strong afferents from limbic structures that can send information about the internal state of the subject (40, 41). The neurophysiological basis of the re-engagement in a sequence of tasks may be based on neuronal activities similar to those found in the ACC that respond in situations that require flexibility to depart from routine behavior (42) or to those from the pre-SMA, which are specifically active before the first movement of a renewed sequence of three movements guided by visual stimuli (no cellular recording of the ACC was done in these tasks) (20). It is unclear from our study whether the ACC activation found for the first task of our triplets reflects re-engagement in a sequence of cognitive decisions or simply re-engagement in a motor sequence.
Encountering the relatively longer inter-trial interval that precedes our first task, relative to those within a triplet, may be experienced and processed as a novel event. Although a novelty effect is likely to be minimized in our study because such trials were not scarce (occurring one-third of the time) and because subjects knew the time course of the experiment after extensive training, this interpretation cannot be ruled out. Indeed, such a novelty effect could be part and parcel of restarting a cognitive activity.
It also could be proposed that the ACC activation may represent a gradual decrease of activation with time. This interpretation is not supported because the ACC was not more activated for the second than the third task of a triplet. Yet another possible interpretation is that the ACC activation reflects unpredictability of the timing of the first task in our triplets. However, this is unlikely because no ACC activation was found in our previous task-switching fMRI study that directly compared random to fixed task timing by using the same tasks as the present work (23). An alternative view of both the ACC and lateral PFC activation may be that they result from an increase of mental effort because of the increased RT observed when re-engaging in a task and when overcoming residual inhibition of a recently performed task. If this were the case, subjects with the greatest activation in these brain regions should show the largest RT/error rate difference. However, post hoc correlations between activation of these brain regions and the corresponding RT/error rate difference were not significant, suggesting that our findings do not solely result from an increase of mental effort.
Comparisons with Previous Task Switching Studies.
Our results provide evidence that task switching can be fractionated into different subprocesses. Unlike previous fMRI studies that could only examine activation for switch and repeat trials, our current study allows us to compare distinct switch trials according to their recency and to focus on the importance of the past on current trials. Distinguishing the neural substrate of re-engagement in a task sequence and of overcoming inhibition of a previously performed task adds to the results from previous fMRI studies examining the neural basis of preparatory component processes involved in task switching (22, 23). Our study also suggests that the pre-SMA/ACC activation previously obtained with task switching (21–23, 43) may be related to the re-engagement in a task, rather than task switching per se. Indeed, in the present work the ACC was also activated when the first task of a sequence was the same task as the last one of a previous triplet.
Conclusion
Taken together, our data demonstrate that the lateral PFC is involved in overcoming residual cognitive inhibition, while the ACC is transiently involved when re-engaging in a sequence of tasks, independently of the lateral PFC. It is interesting to note that the lateral PFC and the ACC belong to two distinct architectonic trends within the frontal lobe (44). The lateral aspect develops later than the medial trend in both ontogeny and phylogeny. This finding suggests that the capacity to overcome inhibition of current decisions may occur later than the ability to re-engage in a sequence of actions, which may represent a more primitive mechanism. Consistent with the importance of the lateral PFC in the development of overcoming residual inhibition, children, infant monkeys and monkeys with lesions of the lateral PFC are impaired in inhibitory control tasks (45). Our results provide challenging avenues for future theories of cognitive control and for better understanding of the complementary functions of the lateral PFC and the ACC, in both normal and pathological neurodevelopment.
Acknowledgments
We thank Philip Kohn and Dr. Shruti Japee for technical assistance.
Abbreviations
fMRI, functional MRI
PFC, prefrontal cortex
ACC, anterior cingulate
RT, response time
BA, Brodmann area
pre-SMA, pre-supplementary motor area
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mayr U. & Keele, S. W. (2000) J. Exp. Psychol. Gen. 129, 4-26. [DOI] [PubMed] [Google Scholar]
- 2.Arbuthnott K. & Frank, J. (2000) Can. J. Exp. Psychol. 54, 33-41. [DOI] [PubMed] [Google Scholar]
- 3.Allport A. & Wylie, G. (2000) in Control of Cognitive Processes: Attention and Performance XVIII, eds. Monsell, S. & Driver, J. (MIT Press, Cambridge, MA), pp. 35–70.
- 4.Gopher D., Armony, L. & Greenshpan, Y. (2000) J. Exp. Psychol. Gen. 129, 308-339. [DOI] [PubMed] [Google Scholar]
- 5.Wylie G. & Allport, A. (2000) Psychol. Res. 63, 212-233. [DOI] [PubMed] [Google Scholar]
- 6.Meiran N., Chorev, Z. & Sapir, A. (2000) Cognit. Psychol. 41, 211-253. [DOI] [PubMed] [Google Scholar]
- 7.Monsell S., Yeung, N. & Azuma, R. (2000) Psychol. Res. 63, 250-264. [DOI] [PubMed] [Google Scholar]
- 8.Mayr U. (2002) Psychol. Bull. Rev. 9, 93-99. [DOI] [PubMed] [Google Scholar]
- 9.Tipper S. P. (2001) Q. J. Exp. Psychol. 54, 321-343. [DOI] [PubMed] [Google Scholar]
- 10.Jonides J., Smith, E. E., Marshuetz, C., Koeppe, R. A. & Reuter-Lorenz, P. A. (1998) Proc. Natl. Acad. Sci. USA 95, 8410-8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Esposito M., Postle, B. R., Jonides, J. & Smith, E. E. (1999) Proc. Natl. Acad. Sci. USA 96, 7514-7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunge S. A., Ochsner, K. N., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. (2001) Brain 124, 2074-2086. [DOI] [PubMed] [Google Scholar]
- 13.Goldman-Rakic P. S. (1996) Philos. Trans. R. Soc. London B 351, 1445-1453. [DOI] [PubMed] [Google Scholar]
- 14.D'Esposito M., Postle, B. R. & Rypma, B. (2000) Exp. Brain Res. 133, 3-11. [DOI] [PubMed] [Google Scholar]
- 15.Kimberg D. Y. & Farah, M. J. (1993) J. Exp. Psychol. Gen. 122, 411-428. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. D., Braver, T. S. & O'Reilly, R. C. (1996) Philos. Trans. R. Soc. London B 351, 1515-1527. [DOI] [PubMed] [Google Scholar]
- 17.Lustig C., May, C. P. & Hasher, L. (2001) J. Exp. Psychol. Gen. 130, 199-207. [DOI] [PubMed] [Google Scholar]
- 18.Devinsky O. & Luciano, D. (1993) in Neurobiology of Cingulate Cortex and Limbic Thalamus, eds. Vogt, B. A. & Gabriel, M. (Birkhauser, Basel), pp. 527–556.
- 19.Paus T. (2001) Nat. Rev. Neurosci. 2, 417-424. [DOI] [PubMed] [Google Scholar]
- 20.Shima K., Mushiake, H., Saito, N. & Tanji, J. (1996) Proc. Natl. Acad. Sci. USA 93, 8694-8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dove A., Pollmann, S., Schubert, T., Wiggins, C. J. & von Cramon, D. Y. (2000) Brain Res. Cognit. Brain Res. 9, 103-109. [DOI] [PubMed] [Google Scholar]
- 22.Sohn M. H., Ursu, S., Anderson, J. R., Stenger, V. A. & Carter, C. S. (2000) Proc. Natl. Acad. Sci. USA 97, 13448-13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreher J. C., Koechlin, E., Ali, S. O. & Grafman, J. (2002) NeuroImage 17, 95-109. [DOI] [PubMed] [Google Scholar]
- 24.Duncan J. & Owen, A. M. (2000) Trends Neurosci. 23, 475-483. [DOI] [PubMed] [Google Scholar]
- 25.Houghton G. & Tipper, S. P. (1996) Brain Cognit. 30, 20-43. [DOI] [PubMed] [Google Scholar]
- 26.Just M. A. & Carpenter, P. A. (1992) Psychol. Rev. 99, 122-149. [DOI] [PubMed] [Google Scholar]
- 27.Miller E. K. & Cohen, J. D. (2001) Annu. Rev. Neurosci. 24, 167-202. [DOI] [PubMed] [Google Scholar]
- 28.Thompson-Schill S. L., D'Esposito, M., Aguirre, G. K. & Farah, M. J. (1997) Proc. Natl. Acad. Sci. USA 94, 14792-14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rugg M. D. & Wilding, E. L. (2000) Trends Cognit. Sci. 4, 108-115. [DOI] [PubMed] [Google Scholar]
- 30.Cohen L., Dehaene, S., Naccache, L., Lehericy, S., Dehaene-Lambertz, G., Henaff, M. A. & Michel, F. (2000) Brain 123, 291-307. [DOI] [PubMed] [Google Scholar]
- 31.Zeki S. & Marini, L. (1998) Brain 121, 1669-1685. [DOI] [PubMed] [Google Scholar]
- 32.Ullsperger M. & von Cramon, D. Y. (2001) NeuroImage 14, 1387-1401. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald A. W., Cohen, J. D., Stenger, V. A. & Carter, C. S. (2000) Science 288, 1835-1838. [DOI] [PubMed] [Google Scholar]
- 34.Gehring W. J. & Knight, R. T. (2000) Nat. Neurosci. 3, 516-520. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. D., Botvinick, M. & Carter, C. S. (2000) Nat. Neurosci. 3, 421-423. [DOI] [PubMed] [Google Scholar]
- 36.Posner M. I. & Petersen, S. E. (1990) Annu. Rev. Neurosci. 13, 25-42. [DOI] [PubMed] [Google Scholar]
- 37.Bush G., Vogt, B. A., Holmes, J., Dale, A. M., Greve, D., Jenike, M. A. & Rosen, B. R. (2002) Proc. Natl. Acad. Sci. USA 99, 523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter C. S., Macdonald, A. M., Botvinick, M., Ross, L. L., Stenger, V. A., Noll, D. & Cohen, J. D. (2000) Proc. Natl. Acad. Sci. USA 97, 1944-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter C. S., Braver, T. S., Barch, D. M., Botvinick, M. M., Noll, D. & Cohen, J. D. (1998) Science 280, 747-749. [DOI] [PubMed] [Google Scholar]
- 40.Pandya D. N., Van Hoesen, G. W. & Mesulam, M. M. (1981) Exp. Brain Res. 42, 319-330. [DOI] [PubMed] [Google Scholar]
- 41.Vogt B. A. & Pandya, D. N. (1987) J. Comp. Neurol. 262, 271-289. [DOI] [PubMed] [Google Scholar]
- 42.Procyk E., Tanaka, Y. L. & Joseph, J. P. (2000) Nat. Neurosci. 3, 502-508. [DOI] [PubMed] [Google Scholar]
- 43.Rushworth M. F., Hadland, K. A., Paus, T. & Sipila, P. K. (2002) J. Neurophysiol. 87, 2577-2592. [DOI] [PubMed] [Google Scholar]
- 44.Pandya D. N. & Barnes, C. L. (1987) in The Frontal Lobes Revisited, ed. Perecman, E. (IRBN Press, New York), pp. 41–68.
- 45.Diamond A. & Goldman-Rakic, P. S. (1989) Exp. Brain Res. 74, 24-40. [DOI] [PubMed] [Google Scholar]