Abstract
Background
Environmental persistent organochlorines (POCs) biomagnify in the food chain, and the chemicals are suspected of being involved in a broad range of human malignancies. It is speculated that some POCs that can interfere with estrogen receptor-mediated responses are involved in the initiation and progression of human breast cancer. The tumor suppressor gene BRCA1 plays a role in cell-cycle control, in DNA repair, and in genomic stability, and it is often downregulated in sporadic mammary cancers. The aim of the present study was to elucidate whether POCs have the potential to alter the expression of BRCA1.
Methods
Using human breast cancer cell lines MCF-7 and MDA-MB-231, the effect on BRCA1 expression of chemicals belonging to different classes of organochlorine chemicals (the pesticide toxaphene, 2,3,7,8-tetrachlorodibenzo-p-dioxin, and three polychlorinated biphenyls [PCB#138, PCB#153 and PCB#180]) was measured by a reporter gene construct carrying 267 bp of the BRCA1 promoter. A twofold concentration range was analyzed in MCF-7, and the results were supported by northern blot analysis of BRCA1 mRNA using the highest concentrations of the chemicals.
Results
All three polychlorinated biphenyls and 2,3,7,8-tetrachlorodibenzo-p-dioxin reduced 17β-estradiol (E2)-induced expression as well as basal reporter gene expression in both cell lines, whereas northern blot analysis only revealed a downregulation of E2-induced BRCA1 mRNA expression in MCF-7 cells. Toxaphene, like E2, induced BRCA1 expression in MCF-7.
Conclusion
The present study shows that some POCs have the capability to alter the expression of the tumor suppressor gene BRCA1 without affecting the cell-cycle control protein p21Waf/Cip1. Some POCs therefore have the potential to affect breast cancer risk.
Keywords: BRCA1, estrogen receptor, polychlorinated biphenyls, tetrachlorodibenzo-p-dioxin, toxaphene
Introduction
The incidence of breast cancer has been increasing steadily over the past 60 years [1], today affecting one in eight women in the United States [2]. Despite tremendous efforts to understand the disease, less than 50% of all cases are of known etiology such as genetic inheritance, first-degree relatives with breast cancer, and age of menstruation and menopause [3].
A large group of lipophilic organochlorines are found to persist in the environment and to biomagnify through the food chain. This group includes pesticides, polychlorinated biphenyls (PCBs) and polychlorinated dibenzo-dioxins/polychlorinated dibenzo-furans [4-8]. Many of these compounds can interfere with a wide range of hormonal responses [9-13], including estrogen receptor (ER)-mediated responses. Due to their lipophilic nature, organochlorines accumulate in the adipose tissue, can cross the placenta and are found in the breast milk [14]. The chemicals give rise to life-long exposure and are supposed to disturb early development. There is, however, a great epidemiological disparity in the literature regarding whether an association exists between POCs and breast cancer [15,16].
The breast cancer susceptibility gene, BRCA1[17], is linked to familial breast cancer [18], and it is found to be downregulated in sporadic breast and ovarian cancers [19,20]. Decreased expression of BRCA1 promotes cell proliferation and can cause cell transformation [20,21]. The function of BRCA1 remains unknown, but strong evidence suggests a role in cell-cycle control and DNA repair [22,23]. Furthermore, BRCA1 expression is regulated by 17β-estradiol (E2) [24,25].
To address whether POCs could affect BRCA1 expression, we studied the effect on BRCA1 promoter activity using a transient reporter gene assay and the effect on the BRCA1 mRNA level by northern blot analysis in two human breast cancer cell lines. Furthermore, the effect on the estrogen-responsive gene (pS2) was studied, as well the effect on the cell-cycle regulator (p21Waf/Cip1), in order to shed some light on the mechanistic pathways involved.
Materials and methods
Chemicals
Toxaphene (technical mixture; Dr Ehrenstorfer, Augsberg, Germany), 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD) (Wellington Laboratories Inc., Guelph, Ontario, Canada), PCB#138, PCB#153 and PCB#180 (Dr Ehrenstorfer), E2 (Sigma-Aldrich Denmark A/S, Copenhagen, Denmark), and ICI 182780 (AstraZeneca, Luton, UK) were used. All compounds were dissolved in ethanol except TCDD, which was dissolved in dimethyl sulfoxide. All concentrations used were nontoxic as revealed by the Cytotoxicity Assay (Promega Corporation, Madison, Wisconsin, USA) and by measurement of total protein (BCA protein reagent; Perbio Science, Helsinborg, Sweden).
Plasmids
The plasmid pGL12 contains 267 bp, spanning -224 to +43 bp of the BRCA1 promoter-α, subcloned upstream of the luciferase gene [26]. The plasmid pHEO1 contains full-length human ERα cDNA [27]. The plasmid pON249 encodes β-galactosidase under control of a CMV promoter [28], and was used as the internal standard for transfection efficiencies.
Reporter gene assay
MCF-7(BUS) cells (ER responsive) and MDA-MB-231 cells (ER nonresponsive) were cultured as described elsewhere [9,29]. For experiments, 7.3 × 105 MCF-7(BUS) cells or 5 × 105 MDA-MB-231 cells were seeded in six-well trays in DMEM (without phenol red) media containing charcoal-depleted fetal bovine serum (Hyclone Europe, Perbio Science, Erembodegem-Aalst, Belgium). MCF-7(BUS) cells were exposed to the given chemicals and transfected with 1.4 g pGL12 and 0.4 g pON249 using 5 μl fugene (Roche, Mannheim, Germany). Cells were harvested in lysis buffer (Roche) 24 hours after exposure and transfection. Luciferase activity was measured using lysis buffer containing 0.5 mM luciferin (Amersham Pharmacia Biotech, Uppsala, Sweden) and 0.5 mM ATP (Sigma). The β-galactosidase activity was measured by a chemiluminescence kit (Roche). MDA-MB-231 cells were exposed and harvested as MCF-7(BUS) but were transfected with 1.5 g pGL12 using 6 μl fugene for 48 hours. Cotransfection with ER was carried out with 0.5 g pHEO1.
Total RNA isolation
On day 1, 2.2 × 106 cells were seeded in 75 cm2 culture flasks and grown for 24 hours in fetal calf serum containing media. The cells were starved in serum-free media on day 2 to obtain G1-synchronization, and they were exposed to chemicals in charcoal-depleted fetal bovine serum media on day 3. Total RNA was harvested after 22 hours of chemical exposure (PUREScript; Gentra Systems, Minneapolis, Minnesota, USA).
Northern blot and hybridization
A BRCA1 probe was produced by PCR on genomic DNA against exon 11 (5'-GAGAGGCATCCAGAAAAGTATCAGG-3' and 5'-CTCTGGGAAAGTATCGCTGTCATG-3') of the BRCA1 gene (882–2216 bp of BRCA1 cDNA [17]). Twenty micrograms of total RNA was used for northern blot analysis (Zeta-probe membrane; Bio-Rad, Hercules, California, USA). The 32P-labeled BRCA1 probe was made by random primed labeling (Megaprime; Amersham Pharmacia Biotech), and was hybridized overnight at 42°C in 50% formamide (as described by the manufacturer). The pS2-specific probe and the internal control (the GADPH probe) were used as described elsewhere [13,29]. The blots were visualized by a PhosphorImager and quantified with ImageQuant (Molecular Dynamics Amersham Biosciences, Sunnyvale, California, USA).
p21Waf/Cip1 protein level
The p21Waf/Cip1 protein was quantified on 24 hour exposure of MCF-7(BUS) to the given chemicals using the Waf1 Elisa kit (Oncogene Research Products, San Diego, California, USA).
Statistics
Statistical analysis was carried out in the Microsoft Excel standard diagram for independent samples test. P values were calculated using the two-tailed t-test two sample, assuming unequal variances.
Results
Reporter gene assay
The dose-response effects of the chemicals on the BRCA1 promoter-luciferase construct (pGL12) in MCF-7(BUS) are presented in Table 1. In MCF-7(BUS), TCDD, ICI 182780, and the PCBs all significantly reduced the basal (ethanol) and the E2-induced promoter activity at the maximum concentrations tested (TCDD, 100 nM; ICI 182780 and PCBs, 10 μM). However, 10 μM toxaphene significantly induced basal promoter activity. TCDD and the pure ER antagonist ICI 182780 had the potential to decrease basal and E2-induced promoter activity at 100 and 10 pM, respectively, while PCB#138 had the potential to decrease E2-induced promoter activity at 100 pM. All chemicals (TCDD, 10 nM; ICI 182780 and PCBs, 10 μM) significantly reduced the BRCA1-promoter activity in MDA-MB-231. Cotransfection of the ER into MDA-MB-231 showed that the BRCA1 promoter is indeed induced by E2 (Table 1); however, only twofold induction occurred compared with the fivefold induction in MCF-7(BUS).
Table 1.
Effect of persistent organochlorines on transient BRCA1-luciferase (pGL12) reporter gene expression in MCF-7(BUS) and MDA-MB-231
| MCF-7(BUS) | Tox.Tech. | Tox.Tech. +100 pM E2 | ICI 182780 | ICI 182780 +100 pM E2 | TCDD | TCDD +100 pM E2 |
| Control | 18 ± 2.9 | 100 ± 11.8 | 18 ± 0.3 | 100 ± 6.3 | 18 ± 0.8 | 100 ± 8.4 |
| 10 μM | 36 ± 8.3* | 59 ± 8.6* | 5 ± 1.1* | 8 ± 3.4* | n.d. | n.d. |
| 1 μM | 20 ± 2.5 | 96 ± 25.2 | 7 ± 0.5* | 7 ± 0.6* | n.d. | n.d. |
| 100 nM | 20 ± 6.8 | 102 ± 16.8 | 5 ± 0.4* | 5 ± 1.7* | 7 ± 0.9* | 46 ± 4.6* |
| 10 nM | 24 ± 8.1 | 95 ± 7.4 | 5 ± 0.7* | 8 ± 2.4* | 7 ± 0.8* | 33 ± 2.6* |
| 1 nM | 23 ± 8.1 | 88 ± 21.8 | 5 ± 0.7* | 46 ± 10.8* | 8 ± 1.3* | 39 ± 3.5* |
| 100 pM | 24 ± 9.3 | 108 ± 11.4 | 9 ± 0.3* | 81 ± 5.0* | 14 ± 2.5* | 66 ± 12.6* |
| 10 pM | 17 ± 1.7 | 112 ± 4.2 | 12 ± 0.8* | 109 ± 25.1 | 16 ± 4.0 | 110 ± 24.1 |
| 1 pM | 21 ± 3.4 | 92 ± 18.9 | 15 ± 2.3 | 101 ± 55.8 | 25 ± 6.7 | 107 ± 13.8 |
| MCF-7(BUS) | PCB#138 | PCB#138 +100 pM E2 | PCB#153 | PCB#153 +100 pM E2 | PCB#180 | PCB#180 +100 pM E2 |
| Control | 18 ± 3.0 | 100 ± 7.8 | 18 ± 2.1 | 100 ± 6.9 | 18 ± 1.7 | 100 ± 12.7 |
| 10 μM | 7 ± 1.2* | 59 ± 14.9* | 8 ± 0.7* | 75 ± 9.0* | 9 ± 3.5* | 70 ± 11.5* |
| 1 μM | 17 ± 1.5 | 81 ± 19.5 | 16 ± 3.5 | 92 ± 17.2 | 13 ± 4.0 | 105 ± 11.7 |
| 100 nM | 16 ± 1.7 | 90 ± 4.9* | 21 ± 0.9 | 97 ± 12.9 | 16 ± 1.4 | 110 ± 24.7 |
| 10 nM | 17 ± 3.9 | 82 ± 10.5* | 22 ± 4.4 | 113 ± 10.6 | 19 ± 5.4 | 107 ± 28.9 |
| 1 nM | 19 ± 3.0 | 87 ± 4.0* | 20 ± 5.1 | 131 ± 28.9 | 23 ± 7.9 | 94 ± 10.2 |
| 100 pM | 18 ± 4.1 | 88 ± 6.5* | 21 ± 6.4 | 95 ± 16.5 | 17 ± 6.4 | 108 ± 31.1 |
| 10 pM | 19 ± 2.2 | 85 ± 21.2 | 22 ± 0.9 | 112 ± 26.7 | 21 ± 7.4 | 112 ± 11.3 |
| 1 pM | 24 ± 5.3 | 86 ± 20.3 | 19 ± 6.2 | 111 ± 9.5 | 16 ± 5.0 | 109 ± 21.2 |
| MDA-MB-231 | Control | Tox.Tech. (10 μM) | TCDD(10 nM) | PCB#138 (10 μM) | PCB#153 (10 μM) | PCB#180 (10 μM) |
| pGL12 | 93 ± 19.3 | 21 ± 8.1* | 65 ± 11.5* | 35 ± 1.3* | 50 ± 10.6* | 49 ± 7.0* |
| pGL12 + 10 nM E2 | 100 ± 15.0 | 21 ± 6.6* | 69 ± 8.6* | 32 ± 6.9* | 53 ± 6.0* | 44 ± 5.6* |
| pGL12/ER | 57 ± 10.4 | 35 ± 11.6* | 55 ± 15.5 | 45 ± 10.6 | 46 ± 12.8 | 48 ± 13.5 |
| pGL12/ER + 10 nM E2 | 100 ± 13.8 | 37 ± 21.3* | 74 ± 35.8 | 65 ± 23.9* | 60 ± 24.5* | 58 ± 36.5 |
All values are corrected with β-galactosidase values (MCF-7(BUS)) or protein (MDA-MB-231) and then normalized to 17β-estradiol (E2)-induced cells (set to 100). The data are mean ± standard deviations of at least five or six measurements from two independent experiments. pGL12/ER, cotransfection with the pHEO1-expressing estrogen receptor alpha; Tox.Tech., toxaphene (technical mixture); TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin; PCB, polychlorinated biphenyl. *P < 0.05 relative to the given control (ethanol/DMSO or E2).
Northern blot analysis
To assess whether the findings in the reporter gene assay are also valid for genomic BRCA1 mRNA expression, northern blot analysis was carried out to measure the effect on the BRCA1 mRNA level with each compound at 10 μM, except for TCDD at 10 nM and ICI 182780 at 100 nM. Figure 1 shows a PhosphorImager image of BRCA1, pS2 and GADPH hybridizations to a northern blot. The analyzed data for BRCA1 are presented in Fig. 2. E2 induced BRCA1 mRNA expression twofold, and all compounds except toxaphene significantly decreased the E2-induced BRCA1 expression in MCF-7. Toxaphene increased the basal BRCA1 mRNA expression, while ICI 182780 downregulated basal and E2-induced expression. No effect of the chemicals on BRCA1 mRNA expression was measured in the ER non-responsive MDA-MB-231 cells.
Figure 1.
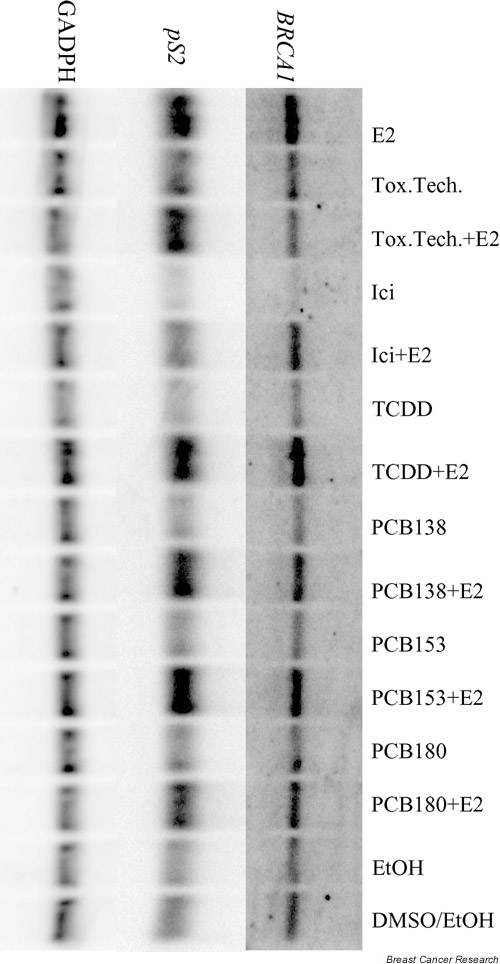
PhosphorImager images of hybridizations with BRCA1 (7.8 kb), pS2 (500 bp), and GADPH (1.3 kb) probes against the same northern blot membrane with 20 μg/lane total RNA from MCF-7. Concentrations of the chemicals are as shown in Fig. 2. The filter represents only one experiment. The collective data are presented in Figs 2 and 3. After hybridization with BRCA1, the filter was stripped and rehybridized with pS2 and then with GADPH. MDA-MB-231 data are not shown. E2, 17β-estradiol; Tox.Tech., toxaphene (technical mixture); Ici, ICI 182780; TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin; PCB, polychlorinated biphenyl; EtOH, ethanol; DMSO, dimethyl sulfoxide.
Figure 2.
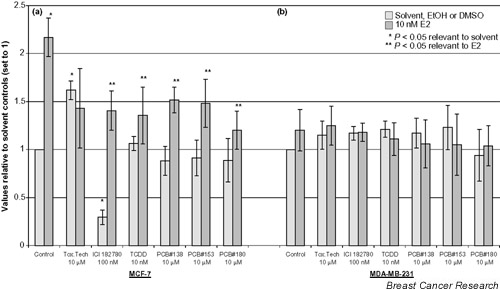
Effect of persistent organochlorines on BRCA1 mRNA expression in (a) MCF-7 and (b) MDA-MB-231. All values are normalized to the internal control (GADPH) and then related to the solvent control (set to 1). Dark bars indicate cotreatment of 17β-estradiol and the given compound. *P < 0.05 compared with solvent-treated cells, **P < 0.05 compared with 17β-estradiol (10 nM)-exposed cells. Data are presented as mean ± standard deviation of at least three independent experiments. E2, 17β-estradiol; Tox.Tech., toxaphene (technical mixture); TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin; PCB, polychlorinated biphenyl; EtOH, ethanol; DMSO, dimethyl sulfoxide.
To further address whether ER mediates the observed deregulation of BRCA1 mRNA, expression of the ER responsive gene pS2 was measured in parallel (Fig. 3a). A 2.5-fold induction of pS2 by E2 was observed, and a twofold induction by toxaphene confirmed its ER agonistic behavior. ICI 182780 significantly decreased basal and E2-induced pS2 expression, whereas TCDD significantly reduced E2-induced pS2 mRNA expression. The three PCBs did not significantly affect the pS2 expression.
Figure 3.
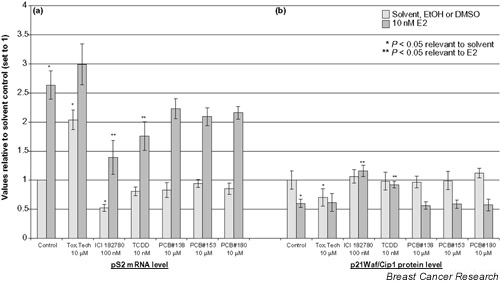
Effect of persistent organochlorines on (a)pS2 mRNA expression in MCF-7 and (b) p21Waf1/Cip1 in MCF-7(BUS). The pS2 data are normalized to GADPH and the related to solvent control (set to 1). Dark bars indicate cotreatment of 17β-estradiol and the given compound. *P < 0.05 compared with solvent-treated cells, **P < 0.05 compared with 17β-estradiol (10 nM)-exposed cells. Data are presented as mean ± standard deviation of at least three independent experiments (pS2) or two independent experiments assayed in duplicate (p21Waf1/Cip1). E2, 17β-estradiol; Tox.Tech., toxaphene (technical mixture); TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin; PCB, polychlorinated biphenyl; EtOH, ethanol; DMSO, dimethyl sulfoxide.
p21Waf/Cip1 study
To address whether the observed response could be a direct consequence of changes in cell-cycle regulation, we determined the effect of the POCs on the negative cell-cycle regulator p21Waf/Cip1. As shown in Fig. 3b, the POCs generally did not affect the p21Waf/Cip1 protein level. Toxaphene, however, decreased the basal p21Waf/Cip1 level like E2. Similar to ICI 182780, TCDD elicited an anti-estrogenic effect on cotreatment with E2.
Discussion
BRCA1 mRNA expression is frequently downregulated in sporadic breast and ovarian cancers [19,20]. This altered regulation is generally not correlated with mutations within the gene [30]. The present study demonstrated that POCs in vitro had the potential to deregulate BRCA1 promoter activity and mRNA expression in human breast cancer cell lines. Using an E2-responsive construct carrying the key positive regulatory element of the BRCA1 promoter upstream of the luciferase gene [26,31], we found that TCDD, PCB#138, PCB#153, and PCB#180 can downregulate the basal as well as the E2-induced BRCA1 promoter activity in both the ER-positive cell line (MCF-7(BUS)) and in the ER-negative cell line (MDA-MB-231). However, toxaphene induced basal promoter activity (Table 1).
The inhibiting effect of TCDD and the three PCBs were confirmed by northern blot analysis on the E2-induced BRCA1 mRNA level in MCF-7 (Fig. 2). E2 induced the BRCA1 mRNA level twofold, and the observed estrogen-like activity of toxaphene supports earlier findings [32,33]. At concentrations of 10 μM, the three PCBs had the capacity to downregulate both basal and E2-induced BRCA1 promoter activity, while they only decreased the E2-induced BRCA1 mRNA level. Furthermore, the PCBs antagonized E2-induced pS2 mRNA expression, but it was not statistically significant (Fig. 3a). The anti-estrogenic potential of the three PCBs mediated via the ER, causing an antiproliferative effect in MCF-7, has previously been published by our laboratory [9].
Jeffy et al. found BRCA1 to be downregulated by benzo [a]pyrene in an ER-dependent fashion, probably through an aryl hydrocarbon receptor-mediated pathway [34]. Using RT-PCR, however, Jeffy et al. did not observe TCDD to downregulate BRCA1 in MCF-7. In the present study, TCDD significantly reduced both BRCA1 promoter activity as well as E2-induced BRCA1 (Fig. 2) and pS2 mRNA expression in MCF-7 (Fig. 3a; see [13]).
All the POCs except toxaphene significantly decreased E2-induced BRCA1 mRNA expression in MCF-7. No effect was measured on the mRNA level in MDA-MB-231 (Fig. 2), which indicates the mechanism of action to be via the ER. In MDA-MB-231, however, the POCs also decreased the BRCA1 promoter activity, suggesting that other pathways than the ER-mediated pathway should be considered. We have shown that the three PCBs in MCF-7(BUS) are antiproliferative and anti-estrogenic at 10 μM [9], as has also been reported for TCDD [34], whereas toxaphene has been shown to increase MCF-7 proliferation [33,35]. In the present study, the negative cell-cycle regulator p21Waf/Cip1 was not affected by the PCBs, while the effect of E2 was as expected [36] and toxaphene decreased the p21Waf/Cip1 protein level. ICI 182780 and TCDD increased the E2 reduced level to its normal level (Fig. 3b). This suggests that the deregulation of BRCA1 observed in this study is not a simple consequence of altered cell-cycle regulation. Since the reporter gene assay also showed an effect of the POCs in MDA-MB-231, further research is needed to elucidate the pathways of the BRCA1 deregulation as well as the involvement of other regulatory sites outside of the 267 bp key regulatory element of the BRCA1 promoter cloned in the pGL-12 vector. In addition, a possible effect on BRCA1 expression of the chemicals in combination and in a mimic of the concentrations found in the adipose tissue should be addressed.
Conclusion
It was found that TCDD, PCB#138, PCB#153, and PCB#180 decreased E2-induced BRCA1 promoter activity and mRNA expression in human breast cancer cell lines. Toxaphene induced the basal BRCA1 expression, probably via inducing cell proliferation since it was found to downregulate the cell-cycle protein p21Waf/Cip1. The presented data show that the ER is likely to play an essential role in the deregulation of BRCA1, but the data indicate a possible involvement of other pathways as well. The effect of these POCs on BRCA1 expression may, however, for a large part be a consequence of their anti-estrogenic, and hence antiproliferative, effect. By deregulating BRCA1, therefore, as has been found in sporadic breast cancers, the POCs could impair the DNA repair machinery, the cell-cycle control and the stress-induced apoptosis [37]. POCs consequently could affect the overall risk of breast cancer.
Abbreviations
bp = base pair; DMEM = Dulbecco's modified Eagle's medium; E2 = 17β-estradiol; ER = estrogen receptor; PCB = polychlorinated biphenyl; PCR = polymerase chain reaction; POC = persistent organochlorine; TCDD = 2,3,7,8 tetrachlorodibenzo-p-dioxin.
Acknowledgments
Acknowledgements
The authors would like to thank Birgitte S Jacobsen for indispensable technical support. pGL12 was a kind gift from E Solomon (UK), and P Chambon (France) provided pHEO1 and the pS2 probe.
References
- Davis DL, Bradlow HL. Can environmental estrogens cause breast cancer? Sci Am. 1995;273:167–172. [PubMed] [Google Scholar]
- Chen X, Danes C, Lowe M, Herliczek TW, Keyomarsi K. Activation of the estrogen-signaling pathway by p21(WAF1/CIP1) in estrogen receptor-negative breast cancer cells. J Natl Cancer Inst. 2000;92:1403–1413. doi: 10.1093/jnci/92.17.1403. [DOI] [PubMed] [Google Scholar]
- Johnson-Thompson MC, Guthrie J. Ongoing research to identify environmental risk factors in breast carcinoma. Cancer. 2000;88(5 suppl):1224–1229. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1224::aid-cncr8>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kidd KA, Schindler DW, Muir DCG, Lockhart WL, Hesslein RH. High concentration of Toxaphene in fishes from a subarctic lake. Science. 1995;269:240–242. doi: 10.1126/science.269.5221.240. [DOI] [PubMed] [Google Scholar]
- Mulvad G, Pedersen HS, Hansen JC, Dewailly E, Jul E, Pederson MB, Bjerregaard P, Malcom GT, Deguchi Y, Middaugh JP. Exposure of Greenlandic Inuit to organochlorines and heavy metals through the marine food-chain: an international study. Sci Total Environ. 1996;186:137–139. doi: 10.1016/0048-9697(96)05093-0. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Toniolo PG. Environmental organochlorine exposure as a potential etiologic factor in breast cancer. Environ Health Perspect. 1995;103(suppl 7):141–145. doi: 10.1289/ehp.95103s7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromberg A, Cleemann M, Carlsen L. Review on persistent organic pollutants in the environment of Greenland and Faroe Islands. Chemosphere. 1999;38:3075–3093. doi: 10.1016/S0045-6535(98)00514-1. [DOI] [PubMed] [Google Scholar]
- Schecter A, Stanley J, Boggess K, Masuda Y, Mes J, Wolff M, Furst P, Furst C, Wilson Yang K, Chisholm B. Polychlorinated biphenyl levels in the tissues of exposed and nonexposed humans. Environ Health Perspect. 1994;102(suppl 1):149–158. doi: 10.1289/ehp.94102s1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen activity. Toxicology. 2001;158:141–153. doi: 10.1016/S0300-483X(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Arcaro KF, Bush B, Niemi WD, Pang S, Vakharia DD. Human health and chemical mixtures: an overview. Environ Health Perspect. 1998;106(suppl 6):1263–1270. doi: 10.1289/ehp.98106s61263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls and human health. Int J Occup Med Environ Health. 1998;11:291–303. [PubMed] [Google Scholar]
- Porterfield SP. Thyroidal dysfunction and environmental chemicals – potential impact on brain development [in process citation]. Environ Health Perspect. 2000;108(suppl 3):433–438. doi: 10.1289/ehp.00108s3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld Jorgensen EC, Autrup H, Hansen JC. Effect of toxaphene on estrogen receptor functions in human breast cancer cells. Carcinogenesis. 1997;18:1651–1654. doi: 10.1093/carcin/18.8.1651. [DOI] [PubMed] [Google Scholar]
- Arctic Monitoring and Assessment Programme AMAP Assessment Report: Arctic Pollution Issues Oslo: Arctic Monitoring and Assessment Programme; 1998.
- Safe SH. Endocrine disruptors and human health – is there a problem? An update. Environ Health Perspect. 2000;108:487–493. doi: 10.1289/ehp.00108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson KJ, Miller AB, Woolcott CG, Sterns EE, McCready DR, Lickley LA, Fish EB, Hiraki GY, Holloway C, Ross T, Hanna WM, SenGupta SK, Weber JP. Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:55–63. [PubMed] [Google Scholar]
- Miki Y, Swensen J, Shattuck Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal J, Hussey C, Tran T, McClure M, Fryre C, Hattier T, Phelps R, Haugen-Strano A, Katcher H, Yakumo K, Gholami Z, Shaffer D, Stone S, Bayer S, Wray C, Bogden R, Dayananth P, Ward J, Tonin P, Narod S, Bristow PK, Norris FH, Helvering L, Morrison P, Rosteck P, Lai M, Barett JC, Lewis C, Neuhausen S, Cannon-Albright L, Goldgar D, Wiseman R, Kamb A, Skolnick MH. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, King MC. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250:1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Zheng W, Luo F, Lu JJ, Baltayan A, Press MF, Zhang ZF, Pike MC. Reduction of BRCA1 expression in sporadic ovarian cancer [see comments]. Gynecol Oncol. 2000;76:294–300. doi: 10.1006/gyno.1999.5664. [DOI] [PubMed] [Google Scholar]
- Thompson ME, Jensen RA, Obermiller PS, Page DL, Holt JT. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995;9:444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- Rao VN, Shao N, Ahmad M, Reddy ES. Antisense RNA to the putative tumor suppressor gene BRCA1 transforms mouse fibroblasts. Oncogene. 1996;12:523–528. [PubMed] [Google Scholar]
- Gowen LC, Avrutskaya AV, Latour AM, Koller BH, Leadon SA. BRCA1 required for transcription-coupled repair of oxidative DNA damage. Science. 1998;281:1009–1012. doi: 10.1126/science.281.5379.1009. [DOI] [PubMed] [Google Scholar]
- MacLachlan TK, Somasundaram K, Sgagias M, Shifman Y, Muschel RJ, Cowan KH, El-Deiry WS. BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J Biol Chem. 2000;275:2777–2785. doi: 10.1074/jbc.275.4.2777. [DOI] [PubMed] [Google Scholar]
- Romagnolo D, Annab LA, Thompson TE, Risinger JI, Terry LA, Barrett JC, Afshari CA. Estrogen upregulation of BRCA1 expression with no effect on localization. Mol Carcinogen. 1998;22:102–109. doi: 10.1002/(sici)1098-2744(199806)22:2<102::aid-mc5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Gudas JM, Nguyen H, Li T, Cowan KH. Hormone-dependent regulation of BRCA1 in human breast cancer cells. Cancer Res. 1995;55:4561–4565. [PubMed] [Google Scholar]
- Xu CF, Chambers JA, Solomon E. Complex regulation of the BRCA1 gene. J Biol Chem. 1997;272:20994–20997. doi: 10.1074/jbc.272.34.20994. [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Cherrington JM, Mocarski ES. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J Virol. 1989;63:1435–1440. doi: 10.1128/jvi.63.3.1435-1440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen EC, Autrup H. Autoregulation of human CYP1A1 gene promotor activity in HepG2 and MCF-7 cells. Carcinogenesis. 1996;17:435–441. doi: 10.1093/carcin/17.3.435. [DOI] [PubMed] [Google Scholar]
- Ozcelik H, To MD, Couture J, Bull SB, Andrulis IL. Preferential allelic expression can lead to reduced expression of BRCA1 in sporadic breast cancers. Int J Cancer. 1998;77:1–6. doi: 10.1002/(SICI)1097-0215(19980703)77:1<1::AID-IJC1>3.3.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Thakur S, Croce CM. Positive regulation of the BRCA1 promoter. J Biol Chem. 1999;274:8837–8843. doi: 10.1074/jbc.274.13.8837. [DOI] [PubMed] [Google Scholar]
- Stelzer A, Chan HM. The relative estrogenic activity of technical toxaphene mixture and two individual congeners. Toxicology. 1999;138:69–80. doi: 10.1016/S0300-483X(99)00093-1. [DOI] [PubMed] [Google Scholar]
- Soto AM, Chung KL, Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994;102:380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffy BD, Schultz EU, Selmin O, Gudas JM, Bowden GT, Romagnolo D. Inhibition of BRCA-1 expression by benzo[a]pyrene and its diol epoxide. Mol Carcinogen. 1999;26:100–118. doi: 10.1002/(sici)1098-2744(199910)26:2<100::aid-mc5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995;103(suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall OW, Carroll JS, Sutherland RL. A low abundance pool of nascent p21WAF1/Cip1 is targeted by estrogen to activate cyclin E*Cdk2. J Biol Chem. 2001;276:45433–45442. doi: 10.1074/jbc.M104752200. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Kaufmann SH, Couch FJ. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J Biol Chem. 2000;275:33487–33496. doi: 10.1074/jbc.M005824200. [DOI] [PubMed] [Google Scholar]


