Abstract
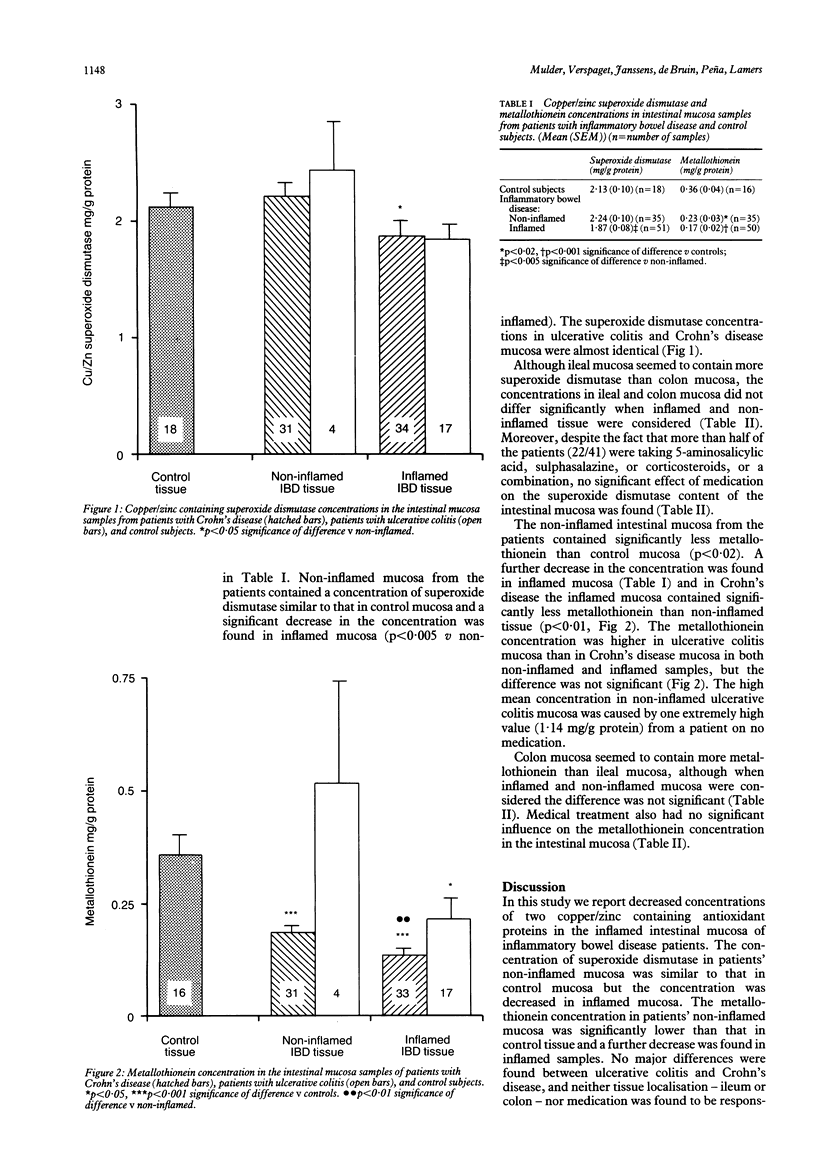
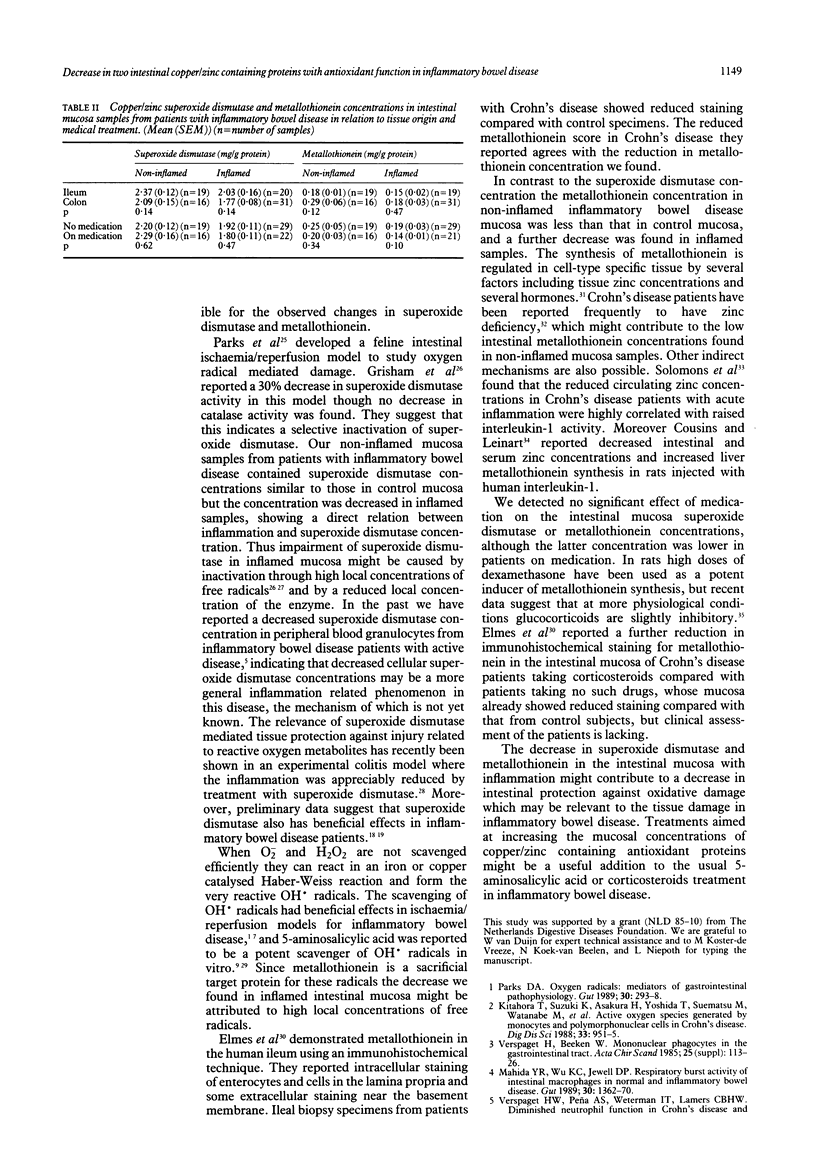
Oxygen derived radicals contribute to tissue injury in inflammatory bowel disease. We measured the content of superoxide dismutase and metallothionein (two endogenous copper and zinc containing proteins involved in radical scavenging) in intestinal resection specimens from 29 patients with Crohn's disease and 12 patients with ulcerative colitis and compared the concentrations with those obtained in the normal mucosa of a control group of 18 patients with colorectal cancer. The superoxide dismutase content was similar in control mucosa and non-inflamed mucosa from patients with inflammatory bowel disease (mean (SEM) 2.13 (0.10) and 2.24 (0.10) mg/g protein, respectively) but was decreased in inflamed mucosa (1.87 (0.08) mg/g protein, p less than 0.005 v non-inflamed mucosa). The metallothionein content was decreased in non-inflamed inflammatory bowel disease mucosa compared with control mucosa (0.23 (0.03) and 0.36 (0.04) mg/g protein, respectively, p less than 0.02) and a further decrease was found in inflamed mucosa (0.17 (0.02) mg/g protein, p less than 0.001 v control mucosa). No differences were found between Crohn's disease and ulcerative colitis and no significant effect of medication or tissue localisation was noted. These findings might indicate a decreased endogenous intestinal protection against oxygen derived radicals in inflammatory bowel disease which could contribute to the pathogenesis of the disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel J., de Ruiter N. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol Lett. 1989 May;47(2):191–196. doi: 10.1016/0378-4274(89)90075-1. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Wasil M., Halliwell B., Hoey B. M., Butler J. The scavenging of oxidants by sulphasalazine and its metabolites. A possible contribution to their anti-inflammatory effects? Biochem Pharmacol. 1987 Nov 1;36(21):3739–3742. doi: 10.1016/0006-2952(87)90028-1. [DOI] [PubMed] [Google Scholar]
- Bakka A., Johnsen A. S., Endresen L., Rugstad H. E. Radioresistance in cells with high content of metallothionein. Experientia. 1982 Mar 15;38(3):381–383. doi: 10.1007/BF01949406. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I. Involvement of metallothionein in the hepatic metabolism of copper. J Nutr. 1987 Jan;117(1):19–29. doi: 10.1093/jn/117.1.19. [DOI] [PubMed] [Google Scholar]
- Cousins R. J., Leinart A. S. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB J. 1988 Oct;2(13):2884–2890. doi: 10.1096/fasebj.2.13.2458983. [DOI] [PubMed] [Google Scholar]
- Crama-Bohbouth G. E., Arndt J. W., Peña A. S., Verspaget H. W., Tjon A Tham R. T., Weterman I. T., Pauwels E. K., Lamers C. B. Value of indium-111 granulocyte scintigraphy in the assessment of Crohn's disease of the small intestine: prospective investigation. Digestion. 1988;40(4):227–236. doi: 10.1159/000199659. [DOI] [PubMed] [Google Scholar]
- Del Soldato P., Campieri M., Brignola C., Bazzocchi G., Gionchetti P., Lanfranchi G. A., Tamba M. A possible mechanism of action of sulfasalazine and 5-aminosalicylic acid in inflammatory bowel diseases: interaction with oxygen free radicals. Gastroenterology. 1985 Nov;89(5):1215–1216. doi: 10.1016/0016-5085(85)90251-3. [DOI] [PubMed] [Google Scholar]
- Dull B. J., Salata K., Van Langenhove A., Goldman P. 5-Aminosalicylate: oxidation by activated leukocytes and protection of cultured cells from oxidative damage. Biochem Pharmacol. 1987 Aug 1;36(15):2467–2472. doi: 10.1016/0006-2952(87)90518-1. [DOI] [PubMed] [Google Scholar]
- Dunn M. A., Blalock T. L., Cousins R. J. Metallothionein. Proc Soc Exp Biol Med. 1987 Jun;185(2):107–119. doi: 10.3181/00379727-185-42525a. [DOI] [PubMed] [Google Scholar]
- Elmes M. E., Clarkson J. P., Jasani B. The immunocytochemical demonstration of metallothionein in human liver and small intestine. Acta Pharmacol Toxicol (Copenh) 1986;59 (Suppl 7):510–513. doi: 10.1111/j.1600-0773.1986.tb02814.x. [DOI] [PubMed] [Google Scholar]
- Emerit J., Pelletier S., Tosoni-Verlignue D., Mollet M. Phase II trial of copper zinc superoxide dismutase (CuZnSOD) in treatment of Crohn's disease. Free Radic Biol Med. 1989;7(2):145–149. doi: 10.1016/0891-5849(89)90005-1. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988 Mar;33(3 Suppl):6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Hendricks K. M., Walker W. A. Zinc deficiency in inflammatory bowel disease. Nutr Rev. 1988 Dec;46(12):401–408. doi: 10.1111/j.1753-4887.1988.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Jewett S. L., Cushing S., Gillespie F., Smith D., Sparks S. Reaction of bovine-liver copper-zinc superoxide dismutase with hydrogen peroxide. Evidence for reaction with H2O2 and HO2- leading to loss of copper. Eur J Biochem. 1989 Apr 1;180(3):569–575. doi: 10.1111/j.1432-1033.1989.tb14683.x. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A., Morgan G., Sedghi S., Gordon J. H., Doria M. Role of reactive oxygen metabolites in experimental colitis. Gut. 1990 Jul;31(7):786–790. doi: 10.1136/gut.31.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahida Y. R., Wu K. C., Jewell D. P. Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel disease. Gut. 1989 Oct;30(10):1362–1370. doi: 10.1136/gut.30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara J. Alteration of radiosensitivity in metallothionein induced mice and a possible role of Zn-Cu-thionein in GSH-peroxidase system. Experientia Suppl. 1987;52:603–612. doi: 10.1007/978-3-0348-6784-9_63. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Miyachi Y., Yoshioka A., Imamura S., Niwa Y. Effect of sulphasalazine and its metabolites on the generation of reactive oxygen species. Gut. 1987 Feb;28(2):190–195. doi: 10.1136/gut.28.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder T. P., Janssens A. R., Verspaget H. W., Lamers C. B. Development of a radioimmunoassay for human metallothionein. J Immunol Methods. 1990 Jul 3;130(2):157–161. doi: 10.1016/0022-1759(90)90043-u. [DOI] [PubMed] [Google Scholar]
- Mulder T. P., Janssens A. R., Verspaget H. W., Lamers C. B. Isolation, characterization, and determination of human liver (copper/zinc) metallothionein. Experientia. 1990 Jul 15;46(7):688–693. doi: 10.1007/BF01939936. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Somiya K., Michelson A. M., Puget K. Effect of liposomal-encapsulated superoxide dismutase on active oxygen-related human disorders. A preliminary study. Free Radic Res Commun. 1985;1(2):137–153. doi: 10.3109/10715768509056547. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Parks D. A. Oxygen radicals: mediators of gastrointestinal pathophysiology. Gut. 1989 Mar;30(3):293–298. doi: 10.1136/gut.30.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomons N. W., Elson C. O., Pekarek R. S., Jacob R. A., Sandstead H. H., Rosenberg I. H. Leukocytic endogenous mediator in Crohn's disease. Infect Immun. 1978 Dec;22(3):637–639. doi: 10.1128/iai.22.3.637-639.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu M., Suzuki M., Miura S., Nagata H., Oshio C., Asakura H., Watanabe M., Tsuchiya M. Sulfasalazine and its metabolites attenuate respiratory burst of leukocytes--a possible mechanism of anti-inflammatory effects. J Clin Lab Immunol. 1987 May;23(1):31–33. [PubMed] [Google Scholar]
- Thornalley P. J., Vasák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985 Jan 21;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- Verspaget H. W., Peña A. S., Weterman I. T., Lamers C. B. Diminished neutrophil function in Crohn's disease and ulcerative colitis identified by decreased oxidative metabolism and low superoxide dismutase content. Gut. 1988 Feb;29(2):223–228. doi: 10.1136/gut.29.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield A. J., Sawyerr A. M., Dhillon A. P., Pittilo R. M., Rowles P. M., Lewis A. A., Pounder R. E. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. 1989 Nov 4;2(8671):1057–1062. doi: 10.1016/s0140-6736(89)91078-7. [DOI] [PubMed] [Google Scholar]



