Abstract
Background
Lysozyme, one of the major protein components of human milk that is also synthesized by a significant percentage of breast carcinomas, is associated with lesions that have a favorable outcome in female breast cancer. Here we evaluate the expression and prognostic value of lysozyme in male breast cancer (MBC).
Methods
Lysozyme expression was examined by immunohistochemical methods in a series of 60 MBC tissue sections and in 15 patients with gynecomastia. Staining was quantified using the HSCORE (histological score) system, which considers both the intensity and the percentage of cells staining at each intensity. Prognostic value of lysozyme was retrospectively evaluated by multivariate analysis taking into account conventional prognostic factors.
Results
Lysozyme immunostaining was negative in all cases of gynecomastia. A total of 27 of 60 MBC sections (45%) stained positively for this protein, but there were clear differences among them with regard to the intensity and percentage of stained cells. Statistical analysis showed that lysozyme HSCORE values in relation to age, tumor size, nodal status, histological grade, estrogen receptor status, metastasis and histological type did not increase the statistical significance. Univariate analysis confirmed that both nodal involvement and lysozyme values were significant predictors of short-term relapse-free survival. Multivariate analysis, according to Cox's regression model, also showed that nodal status and lysozyme levels were significant independent indicators of short-term relapse-free survival.
Conclusion
Tumor expression of lysozyme is associated with lesions that have an unfavorable outcome in male breast cancer. This milk protein may be a new prognostic factor in patients with breast cancer.
Keywords: lysozyme, male breast cancer, prognosis, prolactin receptors
Introduction
Breast cancer is the most frequent malignant tumor in the female population, and represents a leading cause of death in women, with a rising incidence recently in western countries. These carcinomas display a long natural history, and a high variability in biological and clinical behavior. Thus, major efforts have been directed at finding new prognostic factors and therapeutic methods, but breast-cancer-related mortality seems to be stable over time [1-3]. On the other hand, MBC is a very rare disease, with a male-to-female ratio of only 1:99. The incidence pattern of MBC increases logarithmically with age and differs from that seen in women, which shows a decrease at the time of menopause and then increases with age.
The natural history of both tumors is very similar, and histological features seem to be indistinguishable, but epidemiology and hormonal status are very different. Because of the rarity of the disease in men, controlled prospective clinical trials are not feasible; it is unlikely, therefore, that we will ever know as much about MBC as we do about its counterpart in women. The hormonal milieu represents the main difference between both tumors and it is difficult to understand the development of two so histologically similar tumors in such different hormonal environments [4-6].
Lysozyme, discovered by Fleming in 1922 [7], is one of the major protein components of human milk, and has also been detected as a component of the secretion fluid from nonlactating women [8]. This muramidase plays an important role in the primitive nonspecific defense mechanism related to the monocytic-macrophagic system [9,10]. In nonlactating women, Type I secretions (characterized by containing Zn-α2-glycoprotein, apolipoprotein D and gross cystic disease fluid protein-15) are found in most women without breast pathology or with benign breast diseases. Type II secretions contain high concentrations of lactoferrin, lysozyme, and alpha-lactalbumin [11]; this pattern is found in the majority of women studied who have given birth at some point in the previous four years and in a high proportion of oral-contraceptive users. The Type II pattern was also found in a significant percentage (47%) of breast cancer patients, but only in 7% of healthy women after excluding women who have given birth and OCP users [12]. It is also remarkable that this Type II polypeptide pattern is associated with a peak of prolactin secretion after thyroxine-releasing hormone stimulation test in premenopausal nonlactating women [13].
Very recent results from our group have demonstrated positive immunostaining for lysozyme in 69.4% of a series of 177 female breast cancers (FBCs), and it has been associated with factors of favorable outcome such as tumor size, nodal status, and histological grade [14]. Apolipoprotein D and pepsinogen C, two androgen-induced proteins, are also expressed by a significant percentage of male breast carcinomas [15,16]. Both proteins have shown a significant association with factors of favorable outcome in MBC and also a higher expression in MBC when compared with FBC [17,18].
The aim of the present study was to evaluate the expression of lysozyme in MBC and to describe the behavior of this protein as a potential tumor marker in males with breast cancer.
Materials and methods
Study population
This study was performed on a group of 60 males diagnosed with invasive breast cancer between 1979 and 1995 and treated at 17 surgical departments in Spain (listed in the Acknowledgements section). The mean age of the patients was 59.3 years, with a range of 26 to 89 years. Histological grade of tumors was determined according to criteria reported by Bloom and Richardson [19], whereas nodal status was assessed histopathologically. Estrogen receptor content was measured in cytosol extracts using a commercially available kit from Abbott Laboratories (North Chicago, IL, USA). Breast cancers were considered estrogen-receptor-positive if they contained more than 10 fmol/mg total protein.
Surgery was the primary therapeutic method in all patients. The extent of surgery differed according to the surgeon and the institution. Thus, lumpectomy plus axillary dissection was performed in four patients, total mastectomy plus axillary dissection in six cases, modified radical mastectomy in 44 patients, and radical mastectomy in 12 patients. Thirty-four patients received postoperative adjuvant radiotherapy, 31 chemotherapy, and 39 Tamoxifen. Mean follow-up period was 43.9 months, 38.5 months for node-negative tumors, and 44.8 for node-positive carcinomas. During this follow-up period 11 patients developed tumor recurrence, and four of them died of breast cancer. Five patients died of causes unrelated to breast cancer.
We also analyzed the role of lysozyme in benign male breast epithelium, by including in the present study specimens from 15 patients treated for gynecomastia (mean age 42; range 16–69 years).
Methods
Lysozyme purification and antiserum production
Lysozyme was purified from milk of lactating women according to the high performance liquid chromatography procedure. The purity of the obtained antigen was confirmed by automatic Edman degradation after treatment of the protein with pyroglutamate aminopeptidase. Antiserum against the purified protein was raised in New Zealand white rabbits following the method described by Vaitukaitis [20]. The immunized rabbits were bled 6 weeks after protein injection and the obtained serum was dialyzed for 24 hours at 4°C against 20 mM phosphate buffer, pH7.2. Then, the dialyzed material was chromatographed in a column of diethylaminoethyl cellulose equilibrated and eluted in the same phosphate buffer. Finally, the IgG-containing fractions were collected and stored at -20°C until used.
Immunoblot analysis
Samples were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in 13% gels according to the method of Laemmli[21]. The proteins separated by SDS-PAGE were electrophoretically transferred to a Hybond ECL nitrocellulose membrane (Amersham Biosciences, Uppsala, Sweden) at 100 V for 1 hour in a Bio-Rad Trans Blot apparatus (Bio-Rad, Hercules, CA, USA) with a buffer containing 10 mM 3-(cyclohexylamino)-1-propane-sulfonic acid, 4 mM NaOH, and 20% (v/v) methanol. After transfer, the filter was dried, blocked in phosphate-buffered saline solution containing 0.1% Tween 20 and 5% dry powdered milk, and incubated with rabbit antibody against human lysozyme diluted 1/1000 in the same buffer. After 1 hour, the filter was washed three times with 0.1% Tween 20 in phosphate-buffered saline, incubated for 1 hour with peroxidase-labeled donkey antibodies against rabbit immunoglobulins diluted 1/1000, and washed as before. The immunoreactive bands were detected with SuperSignal Chemiluminescent Substrate (Pierce, Milwaukee, WI, USA).
Immunohistochemical staining
Immunohistochemical assays were performed on 6-μm-thick, formalin-fixed, paraffin-embedded tissue sections using the streptavidin-biotin supersensitive method (Bio-genex, San Ramon, CA, USA). Incubation with antiserum against lysozyme (diluted 1/200 in 20 mM phosphate buffer, pH7.2, and 1% bovine seroalbumin) was performed at room temperature for 30 minutes. Then, slides were incubated for 20 minutes with the second biotinylated antibody obtained from Biogenex. Endogenous alkaline phosphatase was blocked with levamisole (diluted 1/50). The reaction with streptavidin-alcalin phosphatase complex reagent (Biogenex) was performed for 20 minutes at room temperature and the reaction was developed with fast red in Tris-buffer, pH7.2 with naphtolphosphate. Later, the slides were contrasted with Mayer hematoxylin and mounted in Aquatex (Merck, Darmstadt, Germany). Specificity of staining was determined using controls that involved incubation of tissue sections with buffer alone or with an equal amount of IgG from non-immunized rabbits. In both cases, there was no significant staining. Furthermore, immunostaining was completely abolished by antiserum preincubation with lysozyme purified from maternal milk as described previously. Tissue sections were scored in a semiquantitative fashion according to the method described by McCarty et al.[22], which considers both the intensity and percentage of cells staining at each intensity. Intensities were classified from 0 (no staining) to 3 (very strong staining), whereas 10% groupings were used for the percentage of cells that stained positive. For each slide, a value designated as HSCORE was obtained after the application of the following algorithm: HSCORE = Σ (I + 1) × PC, where I and PC represent the intensity and the percentage of cells that stained at each intensity, respectively. The immunostained sections were evaluated independently by two pathologists without knowledge of patients' clinical data at the time of review. Reproducibility of the scoring method between two observers was greater than 90%. The agreement between the two observers is with respect to the consideration of positive or negative tumors, not with respect to the HSCORE values. In the remaining cases, in which discrepancies had been noted, differences were settled by consensus review of corresponding slides.
Statistical analysis
Analysis of differences in lysozyme values between two groups of patients was performed with the Mann-Whitney U test. Relationships between more than two groups were evaluated by the Kruskal-Wallis test. Survival curves were calculated using the Kaplan-Meier [23] method, and differences between curves were evaluated with the Log-rank test [24]. Cox's regression model [25] was also used to examine several combinations and interactions of prognostic factors in a multivariate analysis.
The following variables were included in the analysis: age, tumor size, histological grade, nodal status, and estrogen receptor status.
Selection of prognostic variables was performed with Cox's model using the stepwise regression option from BMDP software [26]. Statistical significance was established at the P < 0.05 level.
Results
The specificity of the antibody against human lysozyme was tested by western blot. As can be seen in Fig. 1, the antibody binds a protein with the same electrophoretic mobility as lysozyme in human milk. Thus, the antibody recognizes the lysozyme present in human milk, but does not recognize lysozyme from different species (chicken), nor any other protein present in a tumor cytosol or human serum. This antibody recognizes complete lysozyme, not part of it. Thus, the antibody cannot be blocked by a peptide, and inhibition is only feasible using complete human lysozyme, as shown in Fig. 1. The concentration of the blocking peptide that would be required cannot be stated because it varies depending on the human milk sample used. It should be measured as concentration per volume (mg/cm3), but the lysozyme we analyzed was measured in solid phase (mg/cm2), and they are not comparable.
Figure 1.
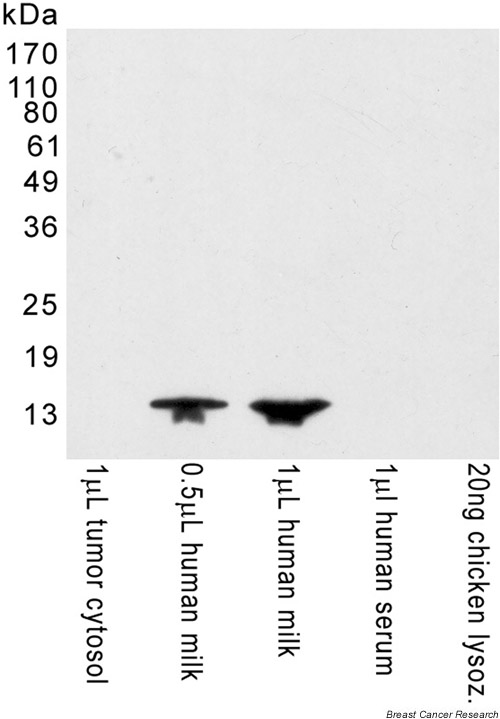
Immunoblot analysis of the specificity of the antibody: the proteins of several samples were separated by SDS-PAGE under reducing conditions, and transferred to a filter. Then, the filter was incubated with antibody against human lysozyme and developed. The proteins recognized by the antibody appear as dark bands against a uniform background. Molecular mass markers are indicated on the left of the gel but not shown in the gel itself. lysoz. = lysozyme.
Immunohistochemical staining of MBCs was also done using controls that involved preincubation, after 30 minutes, of the antibody with human milk. Fig. 2 shows representative examples of these controls.
Figure 2.
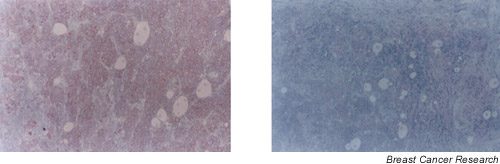
Photomicrographs corresponding to the immunostaining of the same male breast tumour (a) using antilysozyme (×100) and (b) using the same dilution of the antibody previously incubated with human milk (×100).
All 15 specimens from patients with gynecomastia showed lysozyme-negative immunostaining. On the other hand, we did not find normal ducts beside the tumors.
A total of 27 of 60 carcinomas (45%) stained positively for lysozyme, with clear differences among them with regard to intensity and percentage of staining cells. The mean HSCORE value was 85.6.
Tumor characteristics (tumor size, nodal status, metastasis status at the time of diagnosis, histological grade and type and estrogen receptor status) are shown in Table 1. Distribution of lysozyme HSCORE values is shown in Fig. 3. In the group of 27 lysozyme-positive tumors, one tumor was weakly stained (HSCORE<100), 14 were moderately stained (100 <HSCORE<200), and the remaining 12 tumors were strongly positive (HSCORE>200).
Table 1.
Lysozyme HSCORE values classified according to various characteristics of patients and tumors
| HSCORE* | ||||
| Characteristics | n | Mean ± s.e | Median (range) | P |
| Total tumors | 60 | 85.6 ± 13.6 | 0 (0–320) | |
| Age | n.s. | |||
| <60 | 15 | 85.3 ± 28.8 | 0 (0–270) | |
| ≥ 60 | 45 | 85.7 ± 15.6 | 0 (0–320) | |
| Tumor size | n.s. | |||
| T1 | 20 | 77.5 ± 22.7 | 0 (0–270) | |
| T2 | 19 | 110 ± 27.3 | 100 (0–320) | |
| T3–T4 | 21 | 71.4 ± 21.3 | 0 (0–270) | |
| Nodal status | n.s. | |||
| N0 | 32 | 89.6 ± 19.7 | 0 (0–320) | |
| N+ | 28 | 84.1 ± 19.5 | 0 (0–270) | |
| Histological grade | n.s. | |||
| I | 12 | 62.5 ± 34 | 0 (0–320) | |
| II | 28 | 102.8 ± 20.1 | 110 (0–270) | |
| III | 20 | 75.5 ± 21.9 | 0 (0–270) | |
| Metastasis status | n.s. | |||
| M0 | 57 | 87.7 ± 14.2 | 0 (0–320) | |
| M1 | 3 | 46.6 ± 46.6 | 0 (0–140) | |
| Estrogen receptor status (fmol/mg protein) | ||||
| ≥ 10 | 30 | 81.6 ± 18.5 | 0 (0–270) | |
| <10 | 3 | 0 | 0 (0) | |
| Histological type | n.s. | |||
| Ductal | 53 | 93.5 ± 14.8 | 0 (0–320) | |
| Lobular | 2 | 0 | 0 (0) | |
| Papillary | 3 | 60 ± 60 | 0 (0–180) | |
| Cribiform | 2 | 0 | 0 (0) | |
*Semiquantitive immunostaining score for lysozyme. s.e. = standard error; n.s. = not significant.
Figure 3.
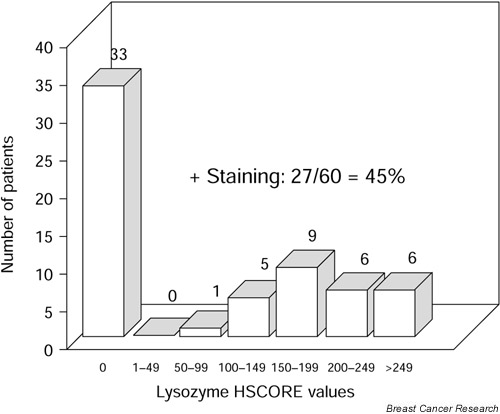
Distribution of HSCORE values obtained by immunohistochemical staining of lysozyme in 60 male breast carcinomas.
The wide variability of lysozyme values obtained suggested a wide variability in biological and clinical behavior of breast carcinomas and the potential value of lysozyme expression as a prognostic marker.
Statistical analysis showed that lysozyme HSCORE values in relation to age, tumor size, nodal status, histological grade, estrogen receptor status, metastasis and histological type did not increase the statistical significance (Table 1). These facts prompted us to evaluate the potential role of lysozyme as an independent prognostic factor in MBC.
The potential association between lysozyme immunostaining and relapse-free survival (RFS) and overall survival was retrospectively evaluated in 57 male patients without metastasis at the time of diagnosis. First, by statistical analysis, we defined an optimal cut-off value of the ability of lysozyme values to predict the RFS of the study population (Fig. 4). Chi-squared analysis led us to define a HSCORE of 100 as the optimal cut-off, with the ability to identify 57.9% of patients as having low or negative lysozyme values (Chi-squared = 5.04; P = 0.0248). Using this cut-off value, relapse was confirmed in 3 of 33 patients (9.10%) with low-level or lysozyme-negative carcinomas, and in 8 of 24 (33.3%) with high-level or lysozyme-positive tumors.
Figure 4.
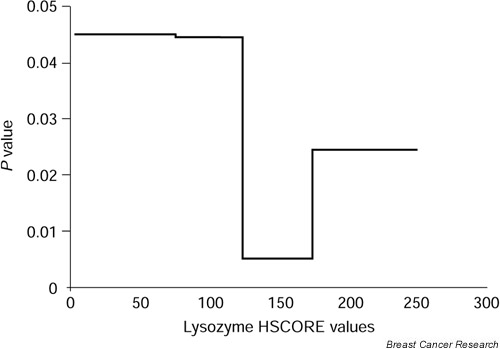
Determination of the cut-off value of lysozyme able to predict RFS in male breast cancer. χ2 values obtained for each cut-off value are plotted against the value itself.
Differences between RFS curves for these two groups of patients were significant at level P < 0.05 (Fig. 5). Similarly, during the study period there was one death (3%) because of recurrence in patients with lysozyme-low or -negative tumors and three deaths (12.5%) in the group of lysozyme-high or -positive tumors. Differences between overall survival curves calculated for these two groups of patients were not significant (Fig. 6).
Figure 5.
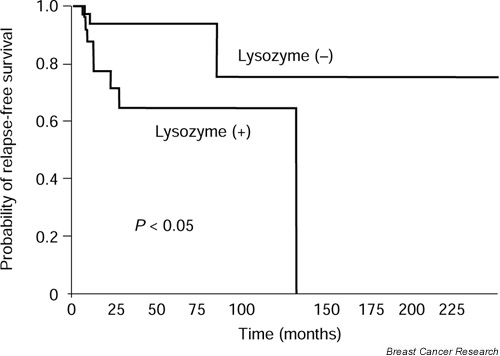
RFS as a function of lysozyme values in 57 males with breast cancer. Differences between curves were significant at P < 0.05.
Figure 6.
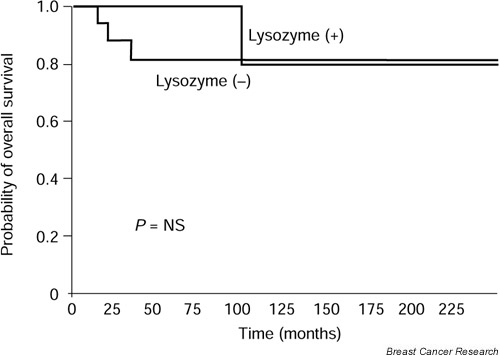
Overall survival as a function of lysozyme values in 57 males with breast cancer patients. Differences between values were not significant.
Univariate analysis confirmed that both nodal involvement (P < 0.005) and high lysozyme values (P < 0.05) were significant predictors of short RFS (Table 2). In addition, multivariate analysis according to Cox's regression model showed that nodal status (P < 0.0005) and high lysozyme levels (P < 0.05) were significant independent indicators of RFS (Table 3).
Table 2.
Univariate analysis of the association of lysozyme with relapse-free survival
| Relapse-free survival (%) | ||||
| Patient and tumor characteristics | n | 5 years (% ± s.e.) | 10 years (% ± s.e.) | P |
| Age | n.s. | |||
| <60 | 16 | 100 | 66 ± 27 | |
| ≥ 60 | 41 | 71 ± 8 | 71 ± 8 | |
| Tumor size | n.s. | |||
| T1 | 20 | 76 ± 12 | 50 ± 2 | |
| T2 | 19 | 94 ± 5 | 94 ± 5 | |
| T3-T4 | 18 | 64 ± 13 | - | |
| Nodal status | <0.005 | |||
| N0 | 32 | 95 ± 4 | 95 ± 4 | |
| N+ | 25 | 61 ± 11 | 41 ± 18 | |
| Histological grade | n.s. | |||
| I | 12 | 100 | - | |
| II | 25 | 81 ± 8 | 81 ± 8 | |
| III | 20 | 68 ± 12 | 41 ± 18 | |
| Lysozyme* | <0.05 | |||
| ≥ 100 | 25 | 64 ± 11 | 64 ± 11 | |
| <100 | 84 | 93 ± 4 | 75 ± 17 | |
*Semiquantitive immunostaining score. s.e. = standard error; n.s. = not significant.
Table 3.
Multivariate analysis of association of lysozyme with relapse-free survival
| Relapse-free survival (%) | |||
| Tumor characteristics | Relative risk | Regression coefficient ± s.e. | P |
| Nodal status | 2.90 ± 1.05 | <0.0005 | |
| N0 | 0.30 | ||
| N+ | 5.50 | ||
| Lysozyme* | -1.37 ± 0.72 | <0.05 | |
| <100 | 0.60 | ||
| ≥ 100 | 2.36 | ||
*Semiquantitive immunostaining score. s.e. = standard error.
Discussion
This is, at present, the first report showing the expression and the prognostic role of lysozyme in MBCs, suggesting that lysozyme could represent a new prognostic indicator of unfavorable outcome in MBC.
The different behavior of lysozyme according to sex of patients is remarkable. Whereas this protein has been detected in 7% of fluid secretions from normal healthy women [12], it is present in 15% of the normal epithelium beside female breast tumors [14]. On the other hand, lysozyme has not been detected in male patients with gynecomastia. Moreover, we did not find normal ducts beside the tumors. Thus, it was impossible to analyze the potential immunostaining of lysozyme in normal male breast ducts due to the fact that, in the normal male breast, gland epithelium does not exist, or is atrophic. But the most important difference shown in the present study has been the opposite meaning of lysozyme secretion in FBC and MBC.
Several biological aspects of lysozyme could contribute to explain the apparent disadvantage conferred by the expression of this protein in MBC and the different meaning of lysozyme in FBC. Lysozyme has been detected in normal female breast tissue and in benign mammary lesions [12] as well as in a subset of female breast tumors (69.4%), in which it has been associated with factors of favorable outcome (histological grade, nodal status, RFS, overall survival) [14]. This association with histological grade may indicate that these female breast tumors possess the required degree of differentiation to synthesize the protein. Milk proteins are synthesized by the mammary epithelium in response to a complex hormonal release during pregnancy and lactation. Although different steroid and peptide hormones cooperate in this process, it is widely accepted that prolactin plays a primary role by increasing transcription of milk genes [27]. Thus, considering that it has been demonstrated that breast cancer cells may show the ability to synthesize a significant amount of biologically active prolactin [28], we may speculate on a potential relationship between lysozyme and prolactin. In addition, several authors have described prolactin receptor expression in about 50% of breast carcinomas [29-33], and shown that receptor expression is more intense in the tumor tissue than in the adjacent normal breast tissue [34].
It is worthwhile mentioning that the optimal cut-off value of lysozyme able to predict RFS in MBC was 100, the same value we established in our study on the role of lysozyme in FBC [14]. Although the prognosis is different in MBC and FBC, this could suggest that this level of lysozyme has a biological value in breast cancer of both sexes.
It is difficult to find a plausible explanation for the different prognostic meaning of lysozyme in MBC. The easiest explanation could be related to the different hormonal milieux. There are more estrogen-receptor-positive tumors in patients with MBC than in FBC [1-6]. Moreover, the higher expression in MBC of other hormonally induced proteins, such as Bcl-2, Zn-α2-glycoprotein, apolipoprotein D, and pepsinogen C, is also well known [17,18].
Another explanation could be that, while the female breast is normally prepared to produce lysozyme, the male breast is not. Thus, its expression by a subset of MBCs may reflect the important transformation undergone by these tissues that results in them behaving as lysozyme-producing carcinomas. This, however, is in contrast with the results of our study, which was unable to show any relationship between lysozyme HSCORE levels and histological grade.
In conclusion, this study demonstrates that lysozyme, a protein normally present in human milk, is expressed by tumor tissue from a significant percentage of males with breast cancer. Moreover, our results show a prognostic significance of lysozyme in MBC with an opposite prognostic meaning of lysozyme in FBC. These controversial results can open new fields in the investigation of the hormonal regulation of breast cancer. Finally, further studies are to be made in order to clarify the induction of lysozyme by prolactin, opening new insights for other modalities of hormonal treatment in breast cancer of both sexes.
Abbreviations
FBC = female breast cancer; HSCORE = histological score; MBC = male breast cancer; RFS = relapse-free survival.
Acknowledgments
Acknowledgements
The authors thank all members of the following participating clinical centers in Spain (listed in alphabetical order): Hospital Virgen de los Lirios de Alcoy, Hospital General de Alicante, Hospital Central de Asturias, Hospital Nuestra Señora de Sonsoles de Ávila, Hospital San Agustín de Avilés, Hospital Virgen de la Luz de Cuenca, Hospital de la Marina Alta de Denia, Hospital General de Elche, Hospital de Elda, Hospital de Jove de Gijón, Hospital de Cabueñes de Gijón, Fundación Jiménez Díaz de Madrid, Hospital Ramón y Cajal de Madrid, Hospital Clínico San Carlos de Madrid, Hospital de la Vega Baja de Orihuela, Centro Médico de Oviedo, Hospital Dr. Peset de Valencia.
We also thank Professor Carlos López-Otín, Departamento de Bio-química, Universidad de Oviedo, Asturias, Spain, for his interesting comments.
References
- Crichlow RW, Galt SW. Male breast cancer. Surg Clin North Am. 1990;70:1165–1177. doi: 10.1016/s0039-6109(16)45237-0. [DOI] [PubMed] [Google Scholar]
- Adami HL, Haulinen T, Ewetz M. The survival pattern in male breast cancer: an analysis of 1429 patients from the Nordic countries. Cancer. 1989;64:1177–1182. doi: 10.1002/1097-0142(19890915)64:6<1177::aid-cncr2820640602>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Heller KS, Rosen PP, Schottenfeld D. Male breast cancer: a clinicopathologic study of 97 cases. Ann Surg. 1978;188:60–65. doi: 10.1097/00000658-197807000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelqvist P, Salmo M. Prognosis of carcinoma of the male breast. Acta Chir Scand. 1982;148:499–502. [PubMed] [Google Scholar]
- Ciatto S, Iossa A, Bonardi R. Male breast carcinoma: review of a multicenter series of 150 cases. Tumori. 1990;76:555–558. doi: 10.1177/030089169007600608. [DOI] [PubMed] [Google Scholar]
- Salvadori B, Saccozzi R, Manzari A, Andreola S, Conti RA, Cusumano F, Grassi M. Prognosis of breast cancer in males: an analysis of 170 cases. Eur J Cancer. 1994;30:930–935. doi: 10.1016/0959-8049(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Fleming A. On a remarkable bacteriolitic element found in tissues and secretions. Proc Roy Soc London. 1922;B93:306–317. [Google Scholar]
- Petrakis NL. Physiologic, biochemical and cytologic aspects of the nipple aspirate fluid. Breast Cancer Res Treat. 1986;8:7–19. doi: 10.1007/BF01805919. [DOI] [PubMed] [Google Scholar]
- Biggar WD, Sturgess JM. Role of lysozyme in the microbicidal activity of rat alveolar macrophages. Infection Immunol. 1977;16:974–982. doi: 10.1128/iai.16.3.974-982.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klokars ML, Robers P. Stimulation of phagocytosis by human lysozyme. Acta Haematol. 1976;52:289–295. doi: 10.1159/000208029. [DOI] [PubMed] [Google Scholar]
- Sánchez LM, Vizoso F, Díez-Itza I, López-Otín C. Identification of the major protein components in breast secretions from women with benign and malignant breast diseases. Cancer Res. 1992;52:95–100. [PubMed] [Google Scholar]
- Vizoso F, Sánchez LM, Díez-Itza I, Lamelas ML, López-Otín C. Factors affecting protein composition of breast secretions from nonlactating women. Breast Cancer Res Treat. 1992;23:251–258. doi: 10.1007/BF01833522. [DOI] [PubMed] [Google Scholar]
- Vizoso F, Díez-Itza I, Sánchez LM, Ruibal A, López-Otín C. Relationship between prolactin levels and composition of breast secretions in nonlactating women. J Clin Endocinol Metab. 1994;79:525–529. doi: 10.1210/jcem.79.2.8045972. [DOI] [PubMed] [Google Scholar]
- Vizoso F, Plaza E, Vázquez J, Serra C, Lamelas ML, González LO, Merino AM, Méndez J. Lysozyme expression by breast carcinomas, correlation with clinicopathologic parameters, and prognostic significance. Ann Surg Oncol. 2001;8:667–674. doi: 10.1007/s10434-001-0667-3. [DOI] [PubMed] [Google Scholar]
- Serra C, Vizoso F, Rodriguez JC, Merino AM, González LO, Baltasar A, Pérez-Vázquez MT, Medrano J. Expression of pepsinogen C in gynecomastias and male breast carcinomas. World J Surg. 1999;23:439–445. doi: 10.1007/pl00012325. [DOI] [PubMed] [Google Scholar]
- Serra C, Vizoso F, Lamelas ML, Rodríguez JC, González LO, Baltasar A, Medrano J. Expression and clinical significance of apolipoprotein D in male breast cancer and gynaecomastia. Br J Surg. 1999;86:1190–1197. doi: 10.1046/j.1365-2168.1999.01157.x. [DOI] [PubMed] [Google Scholar]
- Serra C, Vizoso F, Lamelas ML, Rodríguez JC, González LO, Merino AM, Baltasar A, Pérez-Vázquez MT, Medrano J. Comparative study of two androgen-induced (Apolipoprotein D and Pepsinogen C) prognostic markers between female and male breast carcinoma. Int J Surg Invest. 2000;2:183–192. [PubMed] [Google Scholar]
- Weber-Chappuis K, Bieri-Burger S, Hurliman J. Comparison of prognostic markers detected by immunohistochemistry in male and female breast carcinomas. Eur J Cancer. 1996;32:1686–1692. doi: 10.1016/0959-8049(96)00154-2. [DOI] [PubMed] [Google Scholar]
- Bloom HJG, Richardson WW. Histological grading and prognosis in breast cancer. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis JL. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73:46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Laemmili UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCarty KS, Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, Konrath J, Soper JT, Budwit DA, Creasman WT. Use of a monoclonal anti-estrogen receptor antibody in the inmunohistochemical evaluation of human tumors. Cancer Res. 1986;46:4244–4248. [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Mantel M, Myers M. Problems of convergence of maximum likelihood iterative procedures in muliparameter situations. J Am Stat Assoc. 1971;66:484–491. [Google Scholar]
- Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- Dixon WJ, Brown MB, Engelman L, Frane JW, Hill MA, Jennrich RI. BMDP statistical software. Berkeley: University of California Press; 1985. [Google Scholar]
- Topper YJ, Freeman CS. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1989;60:1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Ginsburg E, Vonderhaar BK. Prolactin synthesis and secretion by human breast cancer cells. Cancer Res. 1995;55:2591–2595. [PubMed] [Google Scholar]
- Holdaway MI, Friesen HG. Hormone binding by human mammary carcinoma. Cancer Res. 1977;37:1946–1952. [PubMed] [Google Scholar]
- Partridge RK, Hahnel R. Prolactin receptors in human breast carcinoma. Cancer. 1979;43:643–646. doi: 10.1002/1097-0142(197902)43:2<643::aid-cncr2820430235>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Turcot Lemay L, Kelly PA. Prolactin in human breast tumors. J Natl Cancer Inst. 1982;68:381–383. [PubMed] [Google Scholar]
- Bonneterre J, Peurat JP, Vandewalle B, BeuscartT R, Vie MC, Cappelaere P. Prolactin receptors in human breast cancer. Eur J Cancer. 1982;18:1157–1162. doi: 10.1016/0277-5379(82)90097-9. [DOI] [PubMed] [Google Scholar]
- Touraine F, Martini JF, Zafrani B, Durand JC, Labaille F, Malet C, Nicolas A, Triven C, Postel-Vinay MC, Kutten F, Kelly PA. Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Met. 1998;83:667–674. doi: 10.1210/jcem.83.2.4564. [DOI] [PubMed] [Google Scholar]
- Peyrat JP, Djiane J, Bonneterre J, Vandewalle B, Vennini PH, Delobelle A, Depadt G, Lefebvre J. Stimulation of DNA synthesis by prolactin in human breast tumor explants. Relation to prolactin receptors. Anticancer Res. 1984;4:257–262. [PubMed] [Google Scholar]


