Abstract
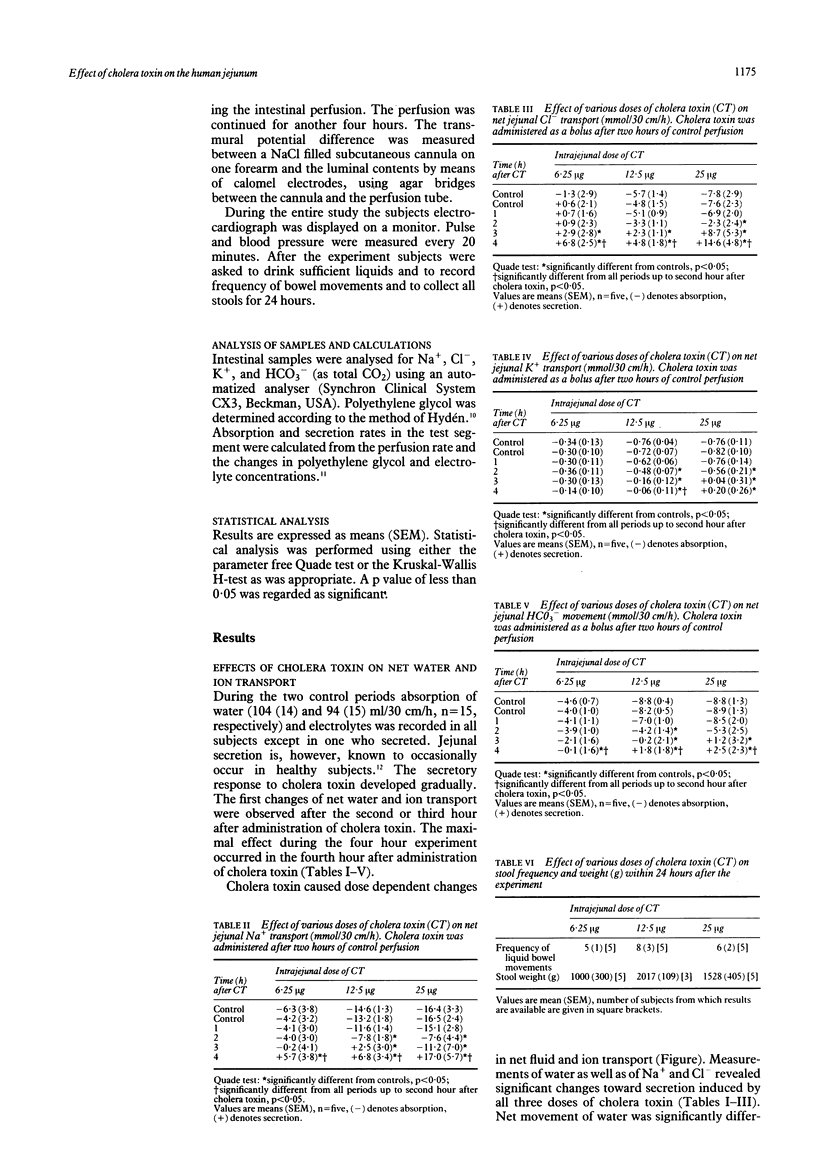
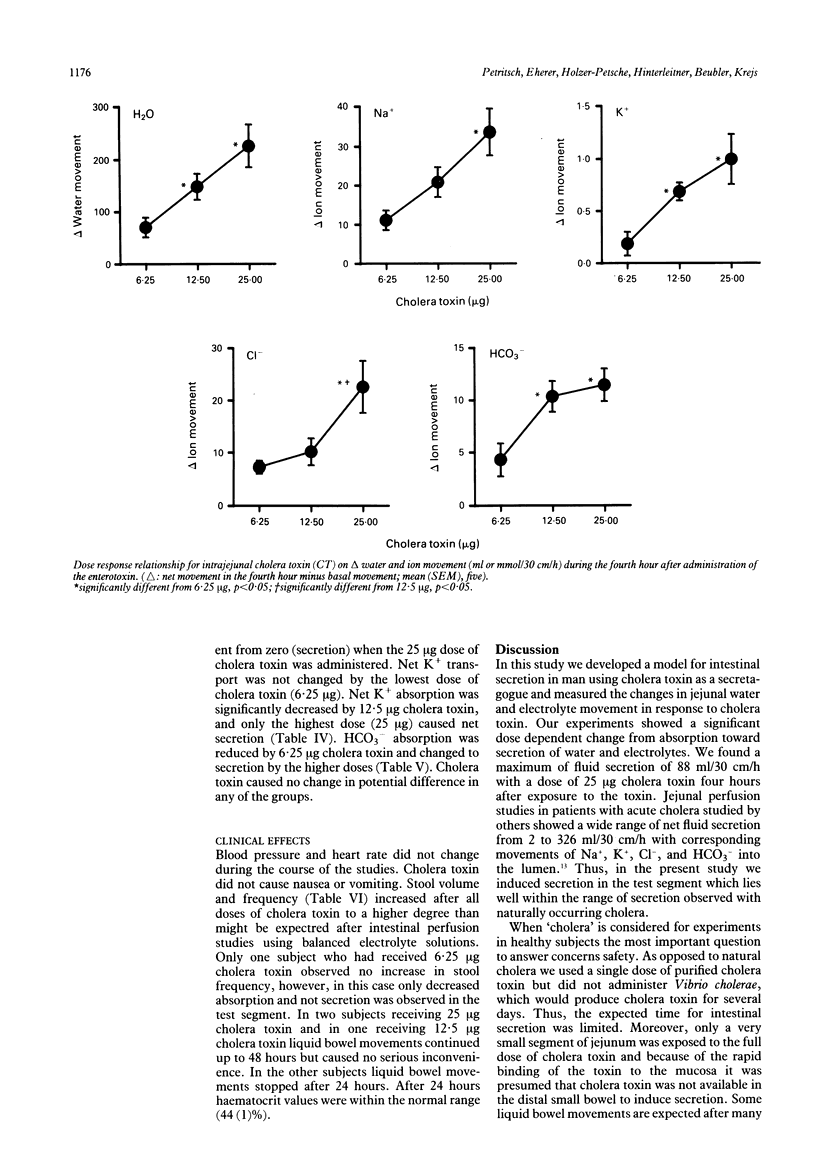
In order to develop a model for secretory diarrhoea and to confirm the in vitro effects of cholera toxin in man in vivo the effect of intrajejunally administered cholera toxin was investigated in healthy volunteers. An intestinal perfusion technique with an occluding balloon proximal to the infusion site was used. The jejunum was perfused under steady state conditions with a plasma like electrolyte solution containing polyethylene glycol as a non-absorbable volume marker. After two control periods of one hour each, during which water was absorbed at a rate of 104 (14) (mean (SEM), n = 15) and 94 (15) ml/30 cm/h, respectively, three different doses of cholera toxin (6.25 micrograms, 12.5 micrograms, 25 micrograms) were administered by bolus into the lumen of the jejunum. Cholera toxin reduced absorption of water and electrolytes progressively over four hours and induced secretion in a dose dependent fashion. In the fourth hour net secretion amounted to 22 (23), 36 (24), and 88 (40) ml/30 cm/h (each n = five) with doses of 6.25, 12.5, and 25 micrograms cholera toxin, respectively. The movement of sodium, chloride, and bicarbonate paralleled water movement. Our results suggest that cholera toxin may serve as a secretory model in the human jejunum which might allow testing of new antisecretory agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz K. M., Mohsin A. K., Hare W. K., Phillips R. A. Using the rat as a cholera "model". Nature. 1968 Nov 23;220(5169):814–815. doi: 10.1038/220814a0. [DOI] [PubMed] [Google Scholar]
- Banwell J. G., Pierce N. F., Mitra R. C., Brigham K. L., Caranasos G. J., Keimowitz R. I., Fedson D. S., Thomas J., Gorbach S. L., Sack R. B. Intestinal fluid and electrolyte transport in human cholera. J Clin Invest. 1970 Jan;49(1):183–195. doi: 10.1172/JCI106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C. Experimental cholera in humans. Br Med J. 1966 Jan 15;1(5480):140–142. doi: 10.1136/bmj.1.5480.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beubler E., Kollar G., Saria A., Bukhave K., Rask-Madsen J. Involvement of 5-hydroxytryptamine, prostaglandin E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology. 1989 Feb;96(2 Pt 1):368–376. doi: 10.1016/0016-5085(89)91560-6. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Greenough W. B., 3rd Response of the canine duodenum to intraluminal challenge with cholera exotoxin. J Clin Invest. 1968 Dec;47(12):2600–2607. doi: 10.1172/JCI105942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. R., Santa Ana C. A., Morawski S., Fordtran J. S. Active chloride secretion in the normal human jejunum. J Clin Invest. 1980 Dec;66(6):1326–1333. doi: 10.1172/JCI109985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer-Petsche U., Petritsch W., Hinterleitner T., Eherer A., Sperk G., Krejs G. J. Effect of neuropeptide Y on jejunal water and ion transport in humans. Gastroenterology. 1991 Aug;101(2):325–330. doi: 10.1016/0016-5085(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Krejs G. J., Barkley R. M., Read N. W., Fordtran J. S. Intestinal secretion induced by vasoactive intestinal polypeptide. A comparison with cholera toxin in the canine jejunum in vivo. J Clin Invest. 1978 May;61(5):1337–1345. doi: 10.1172/JCI109051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejs G. J. Effect of somatostatin infusion on VIP-induced transport changes in the human jejunum. Peptides. 1984 Mar-Apr;5(2):271–276. doi: 10.1016/0196-9781(84)90218-3. [DOI] [PubMed] [Google Scholar]
- Krejs G. J., Fordtran J. S., Fahrenkrug J., Schaffalitzky de Muckadell O. B., Fischer J. E., Humphrey C. S., O'Dorisio T. M., Said S. I., Walsh J. H., Shulkes A. A. Effect of VIP infusion in water and ion transport in the human jejunum. Gastroenterology. 1980 Apr;78(4):722–727. [PubMed] [Google Scholar]
- Leitch G. J., Burrows W. Experimental cholera in the rabbit ligated intestine: ion and water accumulation in the duodenum, ileum and colon. J Infect Dis. 1968 Oct;118(4):349–359. doi: 10.1093/infdis/118.4.349. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuchansky C., Mary J. Y., Bernier J. J. Further studies on prostaglandin E1-induced jejunal secretion of water and electrolytes in man, with special reference to the influence of ethacrynic acid, furosemide, and aspirin. Gastroenterology. 1976 Aug;71(2):274–281. [PubMed] [Google Scholar]
- Modigliani R., Bernier J. J. Absorption of glucose, sodium, and water by the human jejunum studied by intestinal perfusion with a proximal occluding balloon and at variable flow rates. Gut. 1971 Mar;12(3):184–193. doi: 10.1136/gut.12.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modigliani R., Rambaud J. C., Bernier J. J. The method of intraluminal perfusion of the human small intestine. I. Principle and technique. Digestion. 1973;9(2):176–192. doi: 10.1159/000197443. [DOI] [PubMed] [Google Scholar]
- Modigliani R., Rambaud J. C., Bernier J. J. Validation of the use of a tube with a proximal occlusive balloon for measurement of intestinal absorption in man. Am J Dig Dis. 1978 Aug;23(8):720–722. doi: 10.1007/BF01072359. [DOI] [PubMed] [Google Scholar]
- Powell D. W., Binder H. J., Curran P. F. Active electrolyte secretion stimulated by choleragen in rabbit ileum in vitro. Am J Physiol. 1973 Oct;225(4):781–787. doi: 10.1152/ajplegacy.1973.225.4.781. [DOI] [PubMed] [Google Scholar]
- Sachar D. B., Taylor J. O., Saha J. R., Phillips R. A. Intestinal transmural electric potential and its response to glucose in acute and convalescent cholera. Gastroenterology. 1969 Mar;56(3):512–521. [PubMed] [Google Scholar]
- Sladen G. E., Dawson A. M. Further studies on the perfusion method for measuring intestinal absorption in man: the effects of a proximal occlusive balloon and a mixing segment. Gut. 1970 Nov;11(11):947–954. doi: 10.1136/gut.11.11.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantisira M. H., Fändriks L., Jönsson C., Jodal M., Lundgren O. Studies of cholera toxin-induced changes of alkaline secretion and transepithelial potential difference in the rat intestine in vivo. Acta Physiol Scand. 1990 Jan;138(1):75–84. doi: 10.1111/j.1748-1716.1990.tb08814.x. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Fordtran J. S., Carter N. W., Rector F. C., Jr Mechanism of bicarbonate absorption and its relationship to sodium transport in the human jejunum. J Clin Invest. 1970 Mar;49(3):548–556. doi: 10.1172/JCI106265. [DOI] [PMC free article] [PubMed] [Google Scholar]



