Abstract
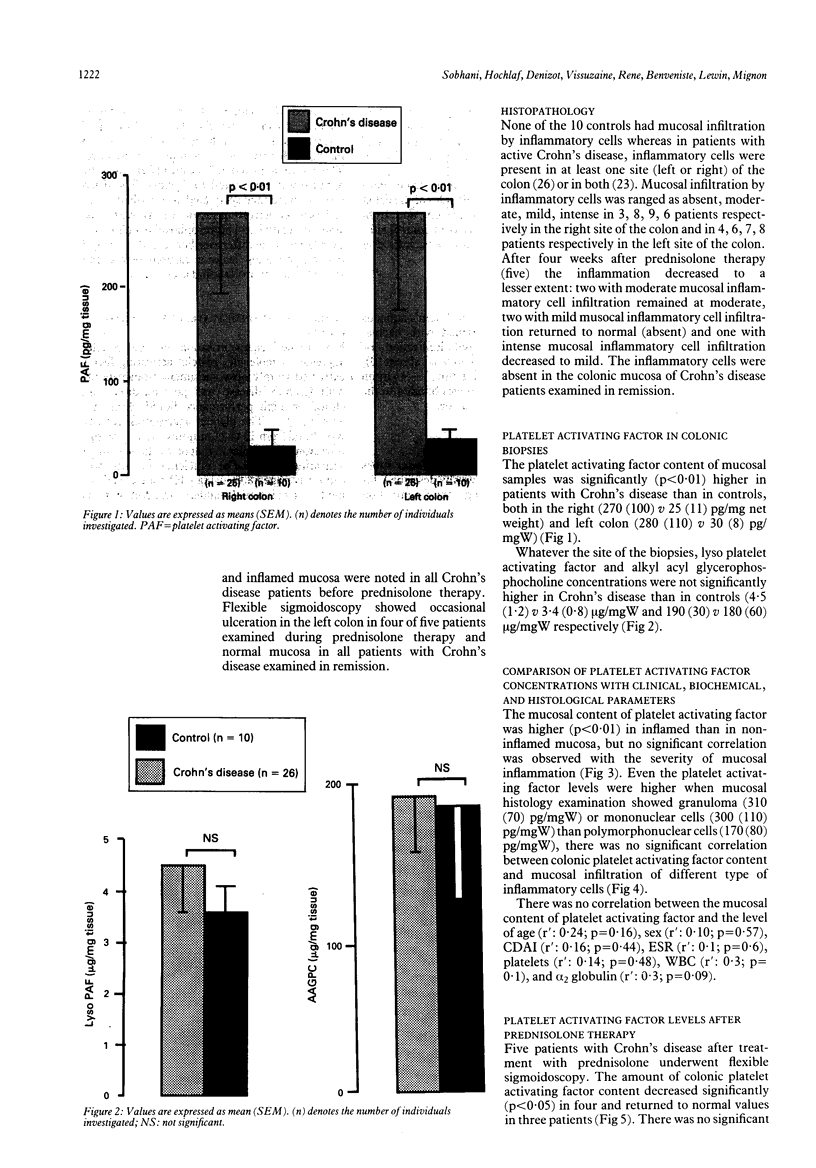
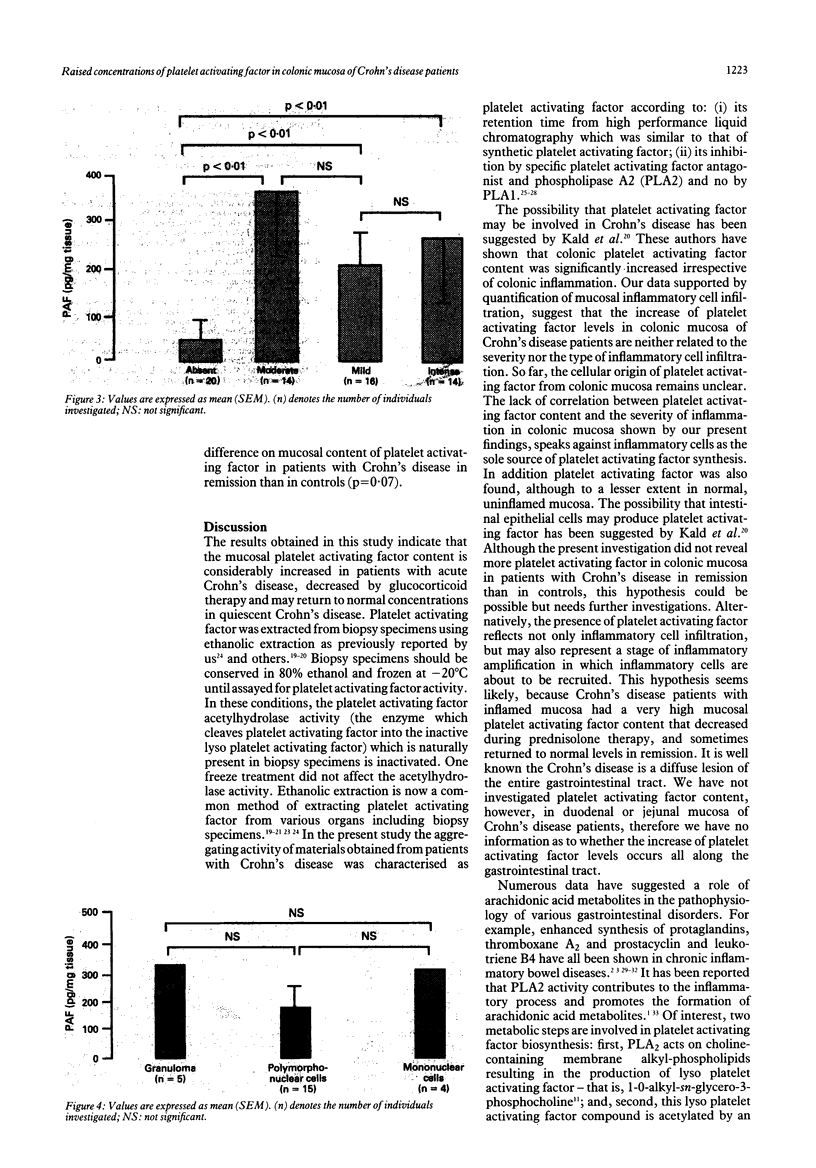
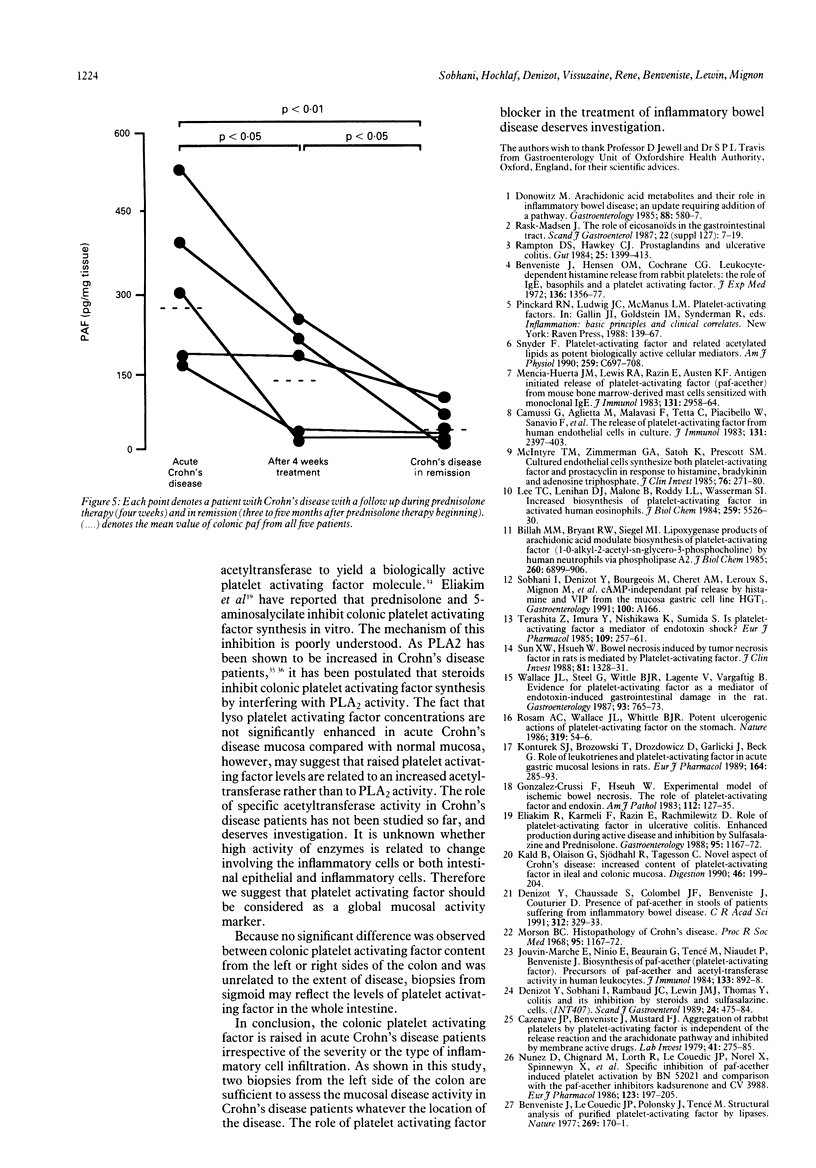
Platelet activating factor (PAF-ACETHER or PAF) and precursors of platelet activating factor were investigated in 26 patients with acute Crohn's disease and in 10 healthy controls. Platelet activating factor, lyso platelet activating factor, and alkyl acyl glycerophosphocholine, were determined in colonic mucosal biopsies in patients with acute Crohn's disease, during prednisolone therapy, and in remission. Biopsy specimens were submitted to histopathology examination and to phospholipid extraction. Platelet activating factor, lyso platelet activating factor, and alkyl acyl glycerophosphocholine were found in patients with acute Crohn's disease and in remission as well as in controls. Whatever the site of the biopsy, the level of platelet activating factor in colonic mucosa was higher (p < 0.01) in Crohn's disease than in controls. There was no correlation between the level of colonic PAF-ACETHER and age, sex, Crohn's disease activity index, and biological parameters in sera. Although concentrations of colonic platelet activating factor content were higher (p < 0.01) when colonic mucosa displayed cell infiltration, they were neither related to the severity nor the type of inflammatory cells. Platelet activating factor decreases with prednisolone therapy and might return to normal concentrations in quiescent patients. Lyso platelet activating factor and alkyl acyl glycerophosphocholine were not significantly higher in Crohn's disease than in controls. These data suggest that platelet activating factor may be involved in the pathogenesis of Crohn's disease and that it could be used as a marker of the mucosal activity of the disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Le Couedic J. P., Polonsky J., Tence M. Structural analysis of purified platelet-activating factor by lipases. Nature. 1977 Sep 8;269(5624):170–171. doi: 10.1038/269170a0. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Bryant R. W., Siegel M. I. Lipoxygenase products of arachidonic acid modulate biosynthesis of platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) by human neutrophils via phospholipase A2. J Biol Chem. 1985 Jun 10;260(11):6899–6906. [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Malavasi F., Tetta C., Piacibello W., Sanavio F., Bussolino F. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983 Nov;131(5):2397–2403. [PubMed] [Google Scholar]
- Cazenave J. P., Benveniste J., Mustard J. F. Aggregation of rabbit platelets by platelet-activating factor is independent of the release reaction and the arachidonate pathway and inhibited by membrane-active drugs. Lab Invest. 1979 Sep;41(3):275–285. [PubMed] [Google Scholar]
- Denizot Y., Chaussade S., Colombel J. F., Benveniste J., Couturier D. Présence du médiateur de l'inflammation paf-acéther dans les selles de patients atteints d'entérocolite inflammatoire. C R Acad Sci III. 1991;312(7):329–333. [PubMed] [Google Scholar]
- Donowitz M. Arachidonic acid metabolites and their role in inflammatory bowel disease. An update requiring addition of a pathway. Gastroenterology. 1985 Feb;88(2):580–587. doi: 10.1016/0016-5085(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Karmeli F., Razin E., Rachmilewitz D. Role of platelet-activating factor in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine and prednisolone. Gastroenterology. 1988 Nov;95(5):1167–1172. doi: 10.1016/0016-5085(88)90346-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Crussi F., Hsueh W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. Am J Pathol. 1983 Jul;112(1):127–135. [PMC free article] [PubMed] [Google Scholar]
- Gustafson C., Tagesson C. Phospholipase activation and arachidonic acid release in cultured intestinal epithelial cells (INT 407). Scand J Gastroenterol. 1989 May;24(4):475–484. doi: 10.3109/00365528909093077. [DOI] [PubMed] [Google Scholar]
- Jouvin-Marche E., Ninio E., Beaurain G., Tence M., Niaudet P., Benveniste J. Biosynthesis of Paf-acether (platelet-activating factor). VII. Precursors of Paf-acether and acetyl-transferase activity in human leukocytes. J Immunol. 1984 Aug;133(2):892–898. [PubMed] [Google Scholar]
- Kald B., Olaison G., Sjödahl R., Tagesson C. Novel aspect of Crohn's disease: increased content of platelet-activating factor in ileal and colonic mucosa. Digestion. 1990;46(4):199–204. doi: 10.1159/000200346. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Drozdowicz D., Garlicki J., Beck G. Role of leukotrienes and platelet activating factor in acute gastric mucosal lesions in rats. Eur J Pharmacol. 1989 May 19;164(2):285–292. doi: 10.1016/0014-2999(89)90469-x. [DOI] [PubMed] [Google Scholar]
- Lee T., Lenihan D. J., Malone B., Roddy L. L., Wasserman S. I. Increased biosynthesis of platelet-activating factor in activated human eosinophils. J Biol Chem. 1984 May 10;259(9):5526–5530. [PubMed] [Google Scholar]
- Ligumsky M., Karmeli F., Sharon P., Zor U., Cohen F., Rachmilewitz D. Enhanced thromboxane A2 and prostacyclin production by cultured rectal mucosa in ulcerative colitis and its inhibition by steroids and sulfasalazine. Gastroenterology. 1981 Sep;81(3):444–449. [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Satoh K., Prescott S. M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985 Jul;76(1):271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Lewis R. A., Razin E., Austen K. F. Antigen-initiated release of platelet-activating factor (PAF-acether) from mouse bone marrow-derived mast cells sensitized with monoclonal IgE. J Immunol. 1983 Dec;131(6):2958–2964. [PubMed] [Google Scholar]
- Ninio E., Mencia-Huerta J. M., Heymans F., Benveniste J. Biosynthesis of platelet-activating factor. I. Evidence for an acetyl-transferase activity in murine macrophages. Biochim Biophys Acta. 1982 Jan 15;710(1):23–31. doi: 10.1016/0005-2760(82)90185-0. [DOI] [PubMed] [Google Scholar]
- Nunez D., Chignard M., Korth R., Le Couedic J. P., Norel X., Spinnewyn B., Braquet P., Benveniste J. Specific inhibition of PAF-acether-induced platelet activation by BN 52021 and comparison with the PAF-acether inhibitors kadsurenone and CV 3988. Eur J Pharmacol. 1986 Apr 16;123(2):197–205. doi: 10.1016/0014-2999(86)90660-6. [DOI] [PubMed] [Google Scholar]
- Olaison G., Leandersson P., Sjödahl R., Tagesson C. Increase in permeability and phospholipase A2 activity of colonic mucosa in Crohn's colitis. Digestion. 1989;43(4):228–233. doi: 10.1159/000199881. [DOI] [PubMed] [Google Scholar]
- Olaison G., Sjödahl R., Tagesson C. Increased phospholipase A2 activity of Ileal mucosa in Crohn's disease. Digestion. 1988;41(3):136–141. doi: 10.1159/000199765. [DOI] [PubMed] [Google Scholar]
- Rampton D. S., Hawkey C. J. Prostaglandins and ulcerative colitis. Gut. 1984 Dec;25(12):1399–1413. doi: 10.1136/gut.25.12.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Madsen J. The role of eicosanoids in the gastrointestinal tract. Scand J Gastroenterol Suppl. 1987;127:7–19. doi: 10.3109/00365528709090945. [DOI] [PubMed] [Google Scholar]
- Rosam A. C., Wallace J. L., Whittle B. J. Potent ulcerogenic actions of platelet-activating factor on the stomach. Nature. 1986 Jan 2;319(6048):54–56. doi: 10.1038/319054a0. [DOI] [PubMed] [Google Scholar]
- Sharon P., Ligumsky M., Rachmilewitz D., Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978 Oct;75(4):638–640. [PubMed] [Google Scholar]
- Sharon P., Stenson W. F. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984 Mar;86(3):453–460. [PubMed] [Google Scholar]
- Snyder F. Platelet-activating factor and related acetylated lipids as potent biologically active cellular mediators. Am J Physiol. 1990 Nov;259(5 Pt 1):C697–C708. doi: 10.1152/ajpcell.1990.259.5.C697. [DOI] [PubMed] [Google Scholar]
- Sun X. M., Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest. 1988 May;81(5):1328–1331. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tencé V., Polonsky J., Le Couedic J. P., Benveniste M. J. Release, purification, and characterization of platelet-activating factor (PAF). Biochimie. 1980;62(4):251–259. doi: 10.1016/s0300-9084(80)80399-3. [DOI] [PubMed] [Google Scholar]
- Terashita Z., Imura Y., Nishikawa K., Sumida S. Is platelet activating factor (PAF) a mediator of endotoxin shock? Eur J Pharmacol. 1985 Feb 26;109(2):257–261. doi: 10.1016/0014-2999(85)90427-3. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Steel G., Whittle B. J., Lagente V., Vargaftig B. Evidence for platelet-activating factor as a mediator of endotoxin-induced gastrointestinal damage in the rat. Effects of three platelet-activating factor antagonists. Gastroenterology. 1987 Oct;93(4):765–773. doi: 10.1016/0016-5085(87)90438-0. [DOI] [PubMed] [Google Scholar]
- Zifroni A., Treves A. J., Sachar D. B., Rachmilewitz D. Prostanoid synthesis by cultured intestinal epithelial and mononuclear cells in inflammatory bowel disease. Gut. 1983 Jul;24(7):659–664. doi: 10.1136/gut.24.7.659. [DOI] [PMC free article] [PubMed] [Google Scholar]


