Abstract
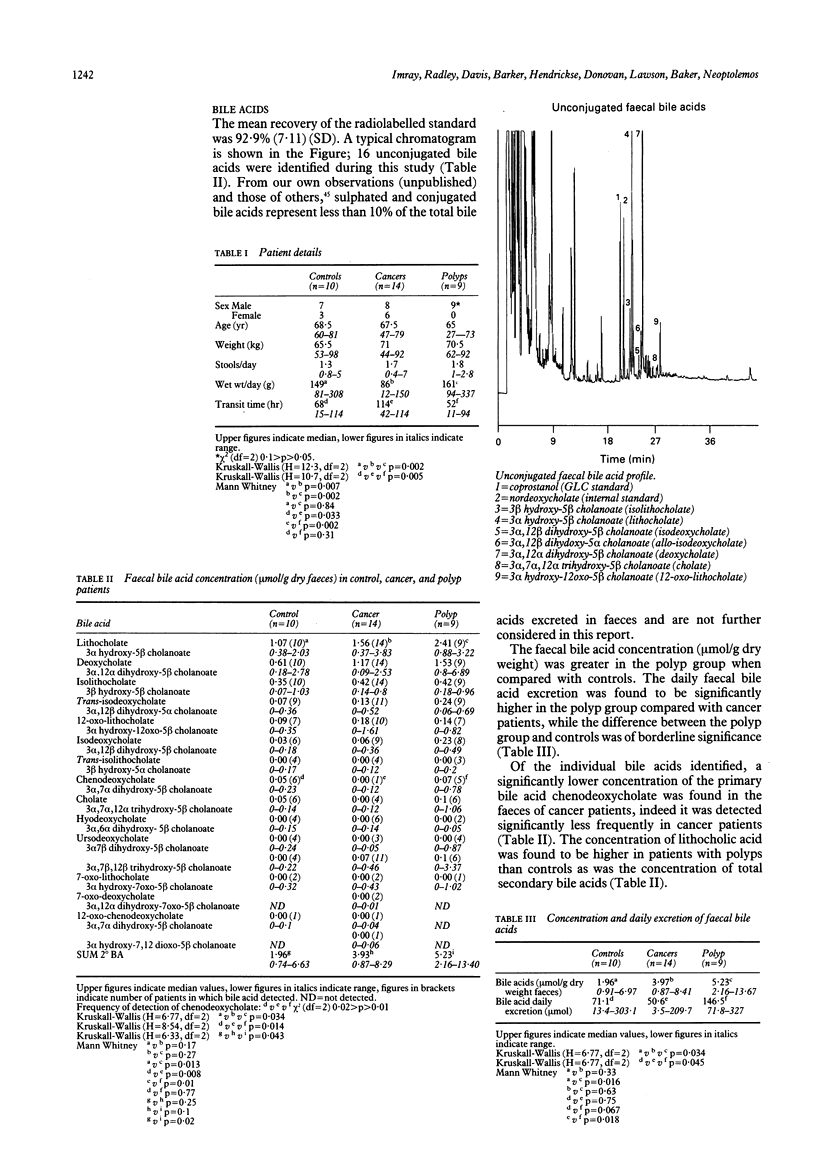
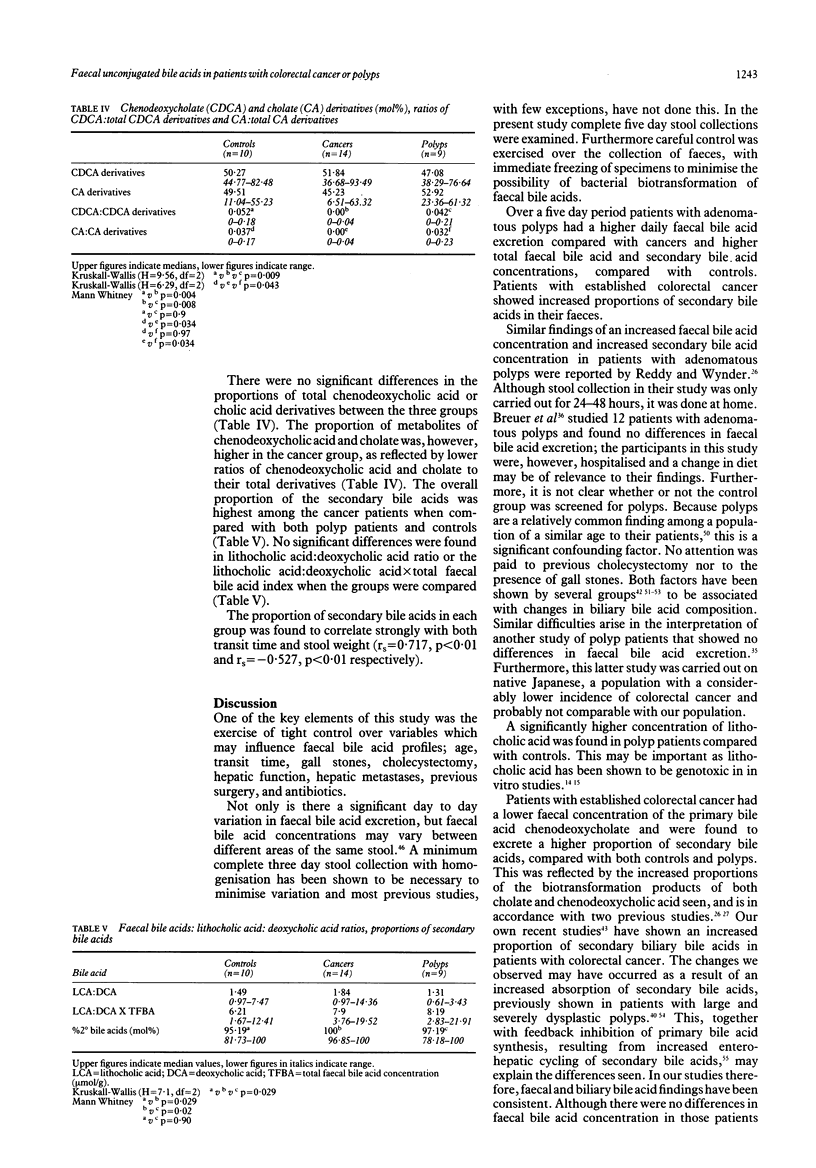
The unconjugated faecal bile acid profiles of 14 patients with colorectal cancer, nine patients with polyps and 10 controls were compared using gas liquid chromatography, controlling for such confounding variables as cholecystectomy, gall stones and hepatic function. Patients with adenomatous polyps had a higher concentration of faecal bile acids (5.23 mumol/g, 2.16-13.67 (median, range) v 1.96, 0.91-6.97; p = 0.016) lithocholic acid (2.41, 0.88-3.22 v 1.07, 0.38-2.03; p = 0.013) and total secondary bile acids (5.23, 2.16-13.4 v 1.96, 0.73-6.63; p = 0.02) compared with control subjects. Patients with colorectal cancer had an increased (p = 0.029) proportion of secondary faecal bile acids (mol%) compared with controls (100, 96.5-100 v 95.19, 81.73-100) and the ratios of the primary bile acids, cholic and chenodeoxycholic acid, to their respective derivatives (secondary bile acids) were significantly lower in cancer patients compared with control and patients with polyps (p = 0.034 to 0.004). This study lends further support to the theory that bile acids may play a role in the development of polyps and colorectal cancer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almé B., Bremmelgaard A., Sjövall J., Thomassen P. Analysis of metabolic profiles of bile acids in urine using a lipophilic anion exchanger and computerized gas-liquid chromatorgaphy-mass spectrometry. J Lipid Res. 1977 May;18(3):339–362. [PubMed] [Google Scholar]
- Aries V., Hill M. J. Degradation of steroids by intestinal bacteria. II. Enzymes catalysing the oxidoreduction of the 3 alpha-, 7 alpha- and 12 alpha-hydroxyl groups in cholic acid, and the dehydroxylation of the 7-hydroxyl group. Biochim Biophys Acta. 1970 May 5;202(3):535–543. doi: 10.1016/0005-2760(70)90124-4. [DOI] [PubMed] [Google Scholar]
- Armstrong B., Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975 Apr 15;15(4):617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- Breuer N. F., Dommes P., Jaekel S., Goebell H. Fecal bile acid excretion pattern in colonic cancer patients. Dig Dis Sci. 1985 Sep;30(9):852–859. doi: 10.1007/BF01309516. [DOI] [PubMed] [Google Scholar]
- Breuer N. F., Jaekel S., Dommes P., Goebell H. Fecal bile acids in patients with adenomatous polyps of the colon. Case-control study. Digestion. 1986;34(2):87–92. doi: 10.1159/000199315. [DOI] [PubMed] [Google Scholar]
- Bull A. W., Marnett L. J., Dawe E. J., Nigro N. D. Stimulation of deoxythymidine incorporation in the colon of rats treated intrarectally with bile acids and fats. Carcinogenesis. 1983;4(2):207–210. doi: 10.1093/carcin/4.2.207. [DOI] [PubMed] [Google Scholar]
- Castleden W. M., Detchon P., Misso N. L. Biliary bile acids in cholelithiasis and colon cancer. Gut. 1989 Jun;30(6):860–865. doi: 10.1136/gut.30.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah P. Y., Bernstein H. Colon cancer and dietary fiber: cellulose inhibits the DNA-damaging ability of bile acids. Nutr Cancer. 1990;13(1-2):51–57. doi: 10.1080/01635589009514044. [DOI] [PubMed] [Google Scholar]
- Cohen B. I., Raicht R. F., Deschner E. E., Takahashi M., Sarwal A. N., Fazzini E. Effect of cholic acid feeding on N-methyl-N-nitrosourea-induced colon tumors and cell kinetics in rats. J Natl Cancer Inst. 1980 Mar;64(3):573–578. [PubMed] [Google Scholar]
- Crowther J. S., Drasar B. S., Hill M. J., Maclennan R., Magnin D., Peach S., Teoh-chan C. H. Faecal steroids and bacteria and large bowel cancer in Hong Kong by socio-economic groups. Br J Cancer. 1976 Aug;34(2):191–198. doi: 10.1038/bjc.1976.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Wiggins H. S., Jenkins D. J., Houston H., Jivraj T., Drasar B. S., Hill M. J. Influence of diets high and low in animal fat on bowel habit, gastrointestinal transit time, fecal microflora, bile acid, and fat excretion. J Clin Invest. 1978 Apr;61(4):953–963. doi: 10.1172/JCI109020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson L. R., Parry J. M. Mitotic aneuploidy as a possible mechanism for tumour promoting activity in bile acids. Carcinogenesis. 1984 Apr;5(4):447–451. doi: 10.1093/carcin/5.4.447. [DOI] [PubMed] [Google Scholar]
- Fisher M. M., Yousef I. M. Sex differences in the bile acid composition of human bile: studies in patients with and without gallstones. Can Med Assoc J. 1973 Aug 4;109(3):190–193. [PMC free article] [PubMed] [Google Scholar]
- Hepner G. W., Hofmann A. F., Malagelada J. R., Szczepanik P. A., Klein P. D. Increased bacterial degradation of bile acids in cholecystectomized patients. Gastroenterology. 1974 Apr;66(4):556–564. [PubMed] [Google Scholar]
- Hikasa Y., Tanida N., Ohno T., Shimoyama T. Faecal bile acid profiles in patients with large bowel cancer in Japan. Gut. 1984 Aug;25(8):833–838. doi: 10.1136/gut.25.8.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S., Hawksworth G., Aries V., Crowther J. S., Williams R. E. Bacteria and aetiology of cancer of large bowel. Lancet. 1971 Jan 16;1(7690):95–100. doi: 10.1016/s0140-6736(71)90837-3. [DOI] [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S., Williams R. E., Meade T. W., Cox A. G., Simpson J. E., Morson B. C. Faecal bile-acids and clostridia in patients with cancer of the large bowel. Lancet. 1975 Mar 8;1(7906):535–539. doi: 10.1016/s0140-6736(75)91556-1. [DOI] [PubMed] [Google Scholar]
- Hinton J. M., Lennard-Jones J. E., Young A. C. A ne method for studying gut transit times using radioopaque markers. Gut. 1969 Oct;10(10):842–847. doi: 10.1136/gut.10.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imray C. H., Davis A., Barker G., Radley S., Baker P., Neoptolemos J. P. Improved human faecal bile acid extraction. Biochem Soc Trans. 1991 Apr;19(2):201S–201S. doi: 10.1042/bst019201s. [DOI] [PubMed] [Google Scholar]
- Kaibara N., Sasaki T., Ikeguchi M., Koga S., Ikawa S. Fecal bile acids and neutral sterols in Japanese with large bowel carcinoma. Oncology. 1983;40(4):255–258. doi: 10.1159/000225738. [DOI] [PubMed] [Google Scholar]
- Kruis W., Forstmaier G., Scheurlen C., Stellaard F. Effect of diets low and high in refined sugars on gut transit, bile acid metabolism, and bacterial fermentation. Gut. 1991 Apr;32(4):367–371. doi: 10.1136/gut.32.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M. S., Cox B. A., Yielding K. L. Requirements for induction of DNA strand breaks by lithocholic acid. Cancer Res. 1982 Jul;42(7):2792–2795. [PubMed] [Google Scholar]
- Malagelada J. R., Go V. L., Summerskill W. H., Gamble W. S. Bile acid secretion and biliary bile acid composition altered by cholecystectomy. Am J Dig Dis. 1973 Jun;18(6):455–459. doi: 10.1007/BF01076595. [DOI] [PubMed] [Google Scholar]
- Moorehead R. J., Campbell G. R., Donaldson J. D., McKelvey S. T. Relationship between duodenal bile acids and colorectal neoplasia. Gut. 1987 Nov;28(11):1454–1459. doi: 10.1136/gut.28.11.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz M., White C., Barnett R. N., Stevens S., Russell E., Vargo D., Floch M. H. Diet, fecal bile acids, and neutral sterols in carcinoma of the colon. Dig Dis Sci. 1979 Oct;24(10):746–751. doi: 10.1007/BF01317206. [DOI] [PubMed] [Google Scholar]
- Mudd D. G., McKelvey S. T., Norwood W., Elmore D. T., Roy A. D. Faecal bile acid concentration of patients with carcinoma or increased risk of carcinoma in the large bowel. Gut. 1980 Jul;21(7):587–590. doi: 10.1136/gut.21.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray W. R., Backwood A., Trotter J. M., Calman K. C., MacKay C. Faecal bile acids and clostridia in the aetiology of colorectal cancer. Br J Cancer. 1980 Jun;41(6):923–928. doi: 10.1038/bjc.1980.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagengast F. M., van der Werf S. D., Lamers H. L., Hectors M. P., Buys W. C., van Tongeren J. M. Influence of age, intestinal transit time, and dietary composition on fecal bile acid profiles in healthy subjects. Dig Dis Sci. 1988 Jun;33(6):673–678. doi: 10.1007/BF01540429. [DOI] [PubMed] [Google Scholar]
- Narisawa T., Magadia N. E., Weisburger J. H., Wynder E. L. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974 Oct;53(4):1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- Owen R. W., Dodo M., Thompson M. H., Hill M. J. Fecal steroids and colorectal cancer. Nutr Cancer. 1987;9(2-3):73–80. doi: 10.1080/01635588709513914. [DOI] [PubMed] [Google Scholar]
- Owen R. W., Henly P. J., Thompson M. H., Hill M. J. Steroids and cancer: faecal bile acid screening for early detection of cancer risk. J Steroid Biochem. 1986 Jan;24(1):391–394. doi: 10.1016/0022-4731(86)90088-9. [DOI] [PubMed] [Google Scholar]
- Rafter J. J., Child P., Anderson A. M., Alder R., Eng V., Bruce W. R. Cellular toxicity of fecal water depends on diet. Am J Clin Nutr. 1987 Mar;45(3):559–563. doi: 10.1093/ajcn/45.3.559. [DOI] [PubMed] [Google Scholar]
- Rampton D. S., Breuer N. F., Vaja S. G., Sladen G. E., Dowling R. H. Role of prostaglandins in bile salt-induced changes in rat colonic structure and function. Clin Sci (Lond) 1981 Nov;61(5):641–648. doi: 10.1042/cs0610641. [DOI] [PubMed] [Google Scholar]
- Reddy B. S. Diet and excretion of bile acids. Cancer Res. 1981 Sep;41(9 Pt 2):3766–3768. [PubMed] [Google Scholar]
- Reddy B. S., Watanabe K., Weisburger J. H., Wynder E. L. Promoting effect of bile acids in colon carcinogenesis in germ-free and conventional F344 rats. Cancer Res. 1977 Sep;37(9):3238–3242. [PubMed] [Google Scholar]
- Reddy B. S., Wynder E. L. Large-bowel carcinogenesis: fecal constituents of populations with diverse incidence rates of colon cancer. J Natl Cancer Inst. 1973 Jun;50(6):1437–1442. doi: 10.1093/jnci/50.6.1437. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Wynder E. L. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977 Jun;39(6):2533–2539. doi: 10.1002/1097-0142(197706)39:6<2533::aid-cncr2820390634>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Redinger R. N. The economy of the enterohepatic circulation of bile acids in the baboon. 2. Regulation of bile acid synthesis by enterohepatic circulation of bile acids. J Lipid Res. 1984 May;25(5):437–447. [PubMed] [Google Scholar]
- Russo P., Taningher M., Pala M., Pisano V., Pedemonte P., De Angeli M. T., Carlone S., Santi L., Parodi S. Characterization of the effects induced on DNA in mouse and hamster cells by lithocholic acid. Cancer Res. 1987 Jun 1;47(11):2866–2874. [PubMed] [Google Scholar]
- Setchell K. D., Ives J. A., Cashmore G. C., Lawson A. M. On the homogeneity of stools with respect to bile acid composition and normal day-to-day variations: a detailed qualitative and quantitative study using capillary column gas chromatography-mass spectrometry. Clin Chim Acta. 1987 Feb 15;162(3):257–275. doi: 10.1016/0009-8981(87)90045-3. [DOI] [PubMed] [Google Scholar]
- Setchell K. D., Lawson A. M., Tanida N., Sjövall J. General methods for the analysis of metabolic profiles of bile acids and related compounds in feces. J Lipid Res. 1983 Aug;24(8):1085–1100. [PubMed] [Google Scholar]
- Tanida N., Hikasa Y., Shimoyama T., Setchell K. D. Comparison of faecal bile acid profiles between patients with adenomatous polyps of the large bowel and healthy subjects in Japan. Gut. 1984 Aug;25(8):824–832. doi: 10.1136/gut.25.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J. R. High colonic pH promotes colorectal cancer. Lancet. 1981 May 16;1(8229):1081–1083. doi: 10.1016/s0140-6736(81)92244-3. [DOI] [PubMed] [Google Scholar]
- Van der Meer R., Welberg J. W., Kuipers F., Kleibeuker J. H., Mulder N. H., Termont D. S., Vonk R. J., De Vries H. T., De Vries E. G. Effects of supplemental dietary calcium on the intestinal association of calcium, phosphate, and bile acids. Gastroenterology. 1990 Dec;99(6):1653–1659. doi: 10.1016/0016-5085(90)90471-c. [DOI] [PubMed] [Google Scholar]
- Watabe J., Bernstein H. The mutagenicity of bile acids using a fluctuation test. Mutat Res. 1985 Oct-Nov;158(1-2):45–51. doi: 10.1016/0165-1218(85)90096-5. [DOI] [PubMed] [Google Scholar]
- Williams A. R., Balasooriya B. A., Day D. W. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982 Oct;23(10):835–842. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilpart M., Mainguet P., Maskens A., Roberfroid M. Structure-activity relationship amongst biliary acids showing comutagenic activity towards 1,2-dimethylhydrazine. Carcinogenesis. 1983 Oct;4(10):1239–1241. doi: 10.1093/carcin/4.10.1239. [DOI] [PubMed] [Google Scholar]
- van Faassen A., Bol J., van Dokkum W., Pikaar N. A., Ockhuizen T., Hermus R. J. Bile acids, neutral steroids, and bacteria in feces as affected by a mixed, a lacto-ovovegetarian, and a vegan diet. Am J Clin Nutr. 1987 Dec;46(6):962–967. doi: 10.1093/ajcn/46.6.962. [DOI] [PubMed] [Google Scholar]
- van der Werf S. D., Nagengast F. M., van Berge Henegouwen G. P., Huijbregts A. W., van Tongeren J. H. Colonic absorption of secondary bile-acids in patients with adenomatous polyps and in matched controls. Lancet. 1982 Apr 3;1(8275):759–762. doi: 10.1016/s0140-6736(82)91810-4. [DOI] [PubMed] [Google Scholar]
- van der Werf S. D., Nagengast F. M., van Berge Henegouwen G. P., Huijbregts A. W., van Tongeren J. H. Intracolonic environment and the presence of colonic adenomas in man. Gut. 1983 Oct;24(10):876–880. doi: 10.1136/gut.24.10.876. [DOI] [PMC free article] [PubMed] [Google Scholar]


