Abstract
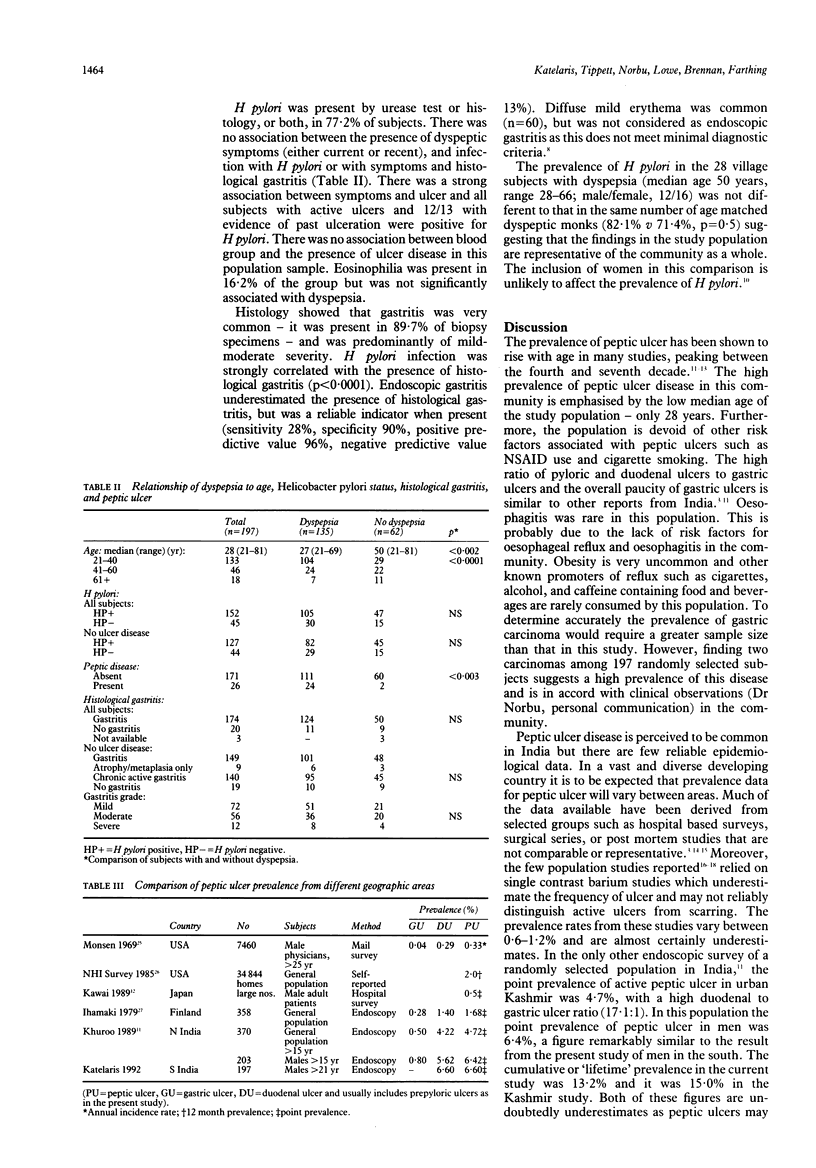
There seems to be a worldwide geographic variation in the prevalence of peptic ulcer disease, although there are few reliable population based studies. This study aimed to determine the prevalence of peptic ulcer disease in a community in southern India and to evaluate the relationship between dyspeptic symptoms, Helicobacter pylori infection, gastritis, and peptic ulcer disease. A sample population was selected randomly from a rural monastic settlement in southern India. Subjects were interviewed using a standardised symptom and demography questionnaire then underwent upper endoscopy and antral biopsy for histology and CLO rapid urease test. Altogether 197 subjects from a population of 1499 (13.1%) were studied. All were male monks and ethnically Tibetan. The median age was 28 years (range: 21-81). None smoked or took NSAIDs. The six month period prevalence of dyspeptic symptoms was 68.5%. Current symptoms were present in 58.9% of subjects. Dyspepsia was more common in subjects aged 40 years or younger (p < 0.0001). H pylori was detected in 77.2% subjects. There was no association between dyspepsia and the presence of H pylori or histological gastritis, although there was a strong correlation between symptoms and ulcer (p < 0.003). The point prevalence of active peptic ulcer was 6.6% (13/197). All ulcers detected were either prepyloric or pyloroduodenal in location. A further 6.6% of subjects had definite evidence of scarring or deformity indicative of ulceration in the past. Subjects with past or present ulcers comprised 17.8% of dyspeptic subjects. H pylori was present in all subjects with active ulcers and in 12/13 of those with scarring. Dyspepsia, H pylori infection, gastritis, and peptic ulcer are all more common in this population than in those from developed countries. Ulcer disease, however, accounts for only a small proportion of subjects with symptoms and neither H pylori infection nor gastritis are significantly associated with the presence of dyspepsia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHATTERJEE S. C., SENGUPTA S. N., DAS D. C. Peptic ulcer in poorer communities of west Bengal: analysis of 100 proved cases. J Indian Med Assoc. 1958 Jan 16;30(2):35–43. [PubMed] [Google Scholar]
- Chuttani C. S., Wig K. L., Chablani T. D., Vasudeva Y. L., Gadekar N. G., Chuttani H. K. Epidemiology of peptic ulcer. I. Prevalence of peptic ulcer in an urban community of Delhi. Indian J Med Res. 1967 Oct;55(10):1121–1128. [PubMed] [Google Scholar]
- Ellard K., Beaurepaire J., Jones M., Piper D., Tennant C. Acute and chronic stress in duodenal ulcer disease. Gastroenterology. 1990 Dec;99(6):1628–1632. doi: 10.1016/0016-5085(90)90467-f. [DOI] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Malaty H. M., Evans D. G., Evans D. J., Jr, Klein P. D., Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991 Jun;100(6):1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- Ihamäki T., Varis K., Siurala M. Morphological, functional and immunological state of the gastric mucosa in gastric carcinoma families. Comparison with a computer-matched family sample. Scand J Gastroenterol. 1979;14(7):801–812. doi: 10.3109/00365527909181408. [DOI] [PubMed] [Google Scholar]
- Kang J. Y., Tay H. H., Wee A., Guan R., Math M. V., Yap I. Effect of colloidal bismuth subcitrate on symptoms and gastric histology in non-ulcer dyspepsia. A double blind placebo controlled study. Gut. 1990 Apr;31(4):476–480. doi: 10.1136/gut.31.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K., Shirakawa K., Misaki F., Hayashi K., Watanabe Y. Natural history and epidemiologic studies of peptic ulcer disease in Japan. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):581–585. doi: 10.1016/s0016-5085(89)80053-8. [DOI] [PubMed] [Google Scholar]
- Khuroo M. S., Mahajan R., Zargar S. A., Javid G., Munshi S. Prevalence of peptic ulcer in India: an endoscopic and epidemiological study in urban Kashmir. Gut. 1989 Jul;30(7):930–934. doi: 10.1136/gut.30.7.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhar R., Siddeshi E. R., Ayyagiri A., Bhasin D. K., Mehta S. K. Campylobacter pylori in dyspeptic subjects: a report from north India. Trans R Soc Trop Med Hyg. 1989 Jan-Feb;83(1):135–135. doi: 10.1016/0035-9203(89)90740-2. [DOI] [PubMed] [Google Scholar]
- Kurata J. H. Ulcer epidemiology: an overview and proposed research framework. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):569–580. doi: 10.1016/s0016-5085(89)80052-6. [DOI] [PubMed] [Google Scholar]
- Lambert J. R., Dunn K., Borromeo M., Korman M. G., Hansky J. Campylobacter pylori--a role in non-ulcer dyspepsia? Scand J Gastroenterol Suppl. 1989;160:7–13. doi: 10.3109/00365528909091728. [DOI] [PubMed] [Google Scholar]
- Loffeld R. J., Potters H. V., Stobberingh E., Flendrig J. A., van Spreeuwel J. P., Arends J. W. Campylobacter associated gastritis in patients with non-ulcer dyspepsia: a double blind placebo controlled trial with colloidal bismuth subcitrate. Gut. 1989 Sep;30(9):1206–1212. doi: 10.1136/gut.30.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALHOTRA S. L., SAIGAL O. N., MODY G. D. ROLE OF SALIVA IN THE AETIOLOGY OF PEPTIC ULCER. Br Med J. 1965 May 8;1(5444):1220–1222. doi: 10.1136/bmj.1.5444.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S. L. Epidemiological study of peptic ulcer in the south of India. Observations from Madras on the changing incidence of peptic ulcer, with special reference to causation. Gut. 1967 Apr;8(2):180–188. doi: 10.1136/gut.8.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanivadekar S. A., Sawant P. D., Saraswathi K., Shroff C. P., Bichile L. S., Patel H. D., Shroff D. S. Association of Campylobacter pylori with gastritis, duodenal ulcer and gastric ulcer--a preliminary report of dyspeptic patients. Indian J Gastroenterol. 1988 Jul;7(3):141–142. [PubMed] [Google Scholar]
- Ostensen H., Gudmundsen T. E., Bolz K. D., Burhol P. G., Bonnevie O. The incidence of gastric ulcer and duodenal ulcer in north Norway. A prospective epidemiological study. Scand J Gastroenterol. 1985 Mar;20(2):189–192. doi: 10.3109/00365528509089655. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Price A. B. The Sydney System: histological division. J Gastroenterol Hepatol. 1991 May-Jun;6(3):209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- RAGHAVAN P. Epidemiology and clinical behavior of peptic ulcer in Bombay, India. Gastroenterology. 1962 Feb;42:130–143. [PubMed] [Google Scholar]
- Rokkas T., Pursey C., Uzoechina E., Dorrington L., Simmons N. A., Filipe M. I., Sladen G. E. Non-ulcer dyspepsia and short term De-Nol therapy: a placebo controlled trial with particular reference to the role of Campylobacter pylori. Gut. 1988 Oct;29(10):1386–1391. doi: 10.1136/gut.29.10.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A. K., Chhuttani P. N., Gupta B. B., Malik K., Gupta H. D. Epidemiology of peptic ulcer in an urban community in Chandigarh. Indian J Med Res. 1971 Oct;59(10):1612–1620. [PubMed] [Google Scholar]
- Talley N. J., Fung L. H., Gilligan I. J., McNeil D., Piper D. W. Association of anxiety, neuroticism, and depression with dyspepsia of unknown cause. A case-control study. Gastroenterology. 1986 Apr;90(4):886–892. doi: 10.1016/0016-5085(86)90864-4. [DOI] [PubMed] [Google Scholar]
- Tibblin G. Introduction to the epidemiology of dyspepsia. Scand J Gastroenterol Suppl. 1985;109:29–33. doi: 10.3109/00365528509103932. [DOI] [PubMed] [Google Scholar]
- Tovey F. Peptic ulcer in India and Bangladesh. Gut. 1979 Apr;20(4):329–347. doi: 10.1136/gut.20.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat G. N. The Sydney System: endoscopic division. Endoscopic appearances in gastritis/duodenitis. J Gastroenterol Hepatol. 1991 May-Jun;6(3):223–234. doi: 10.1111/j.1440-1746.1991.tb01469.x. [DOI] [PubMed] [Google Scholar]



