Abstract
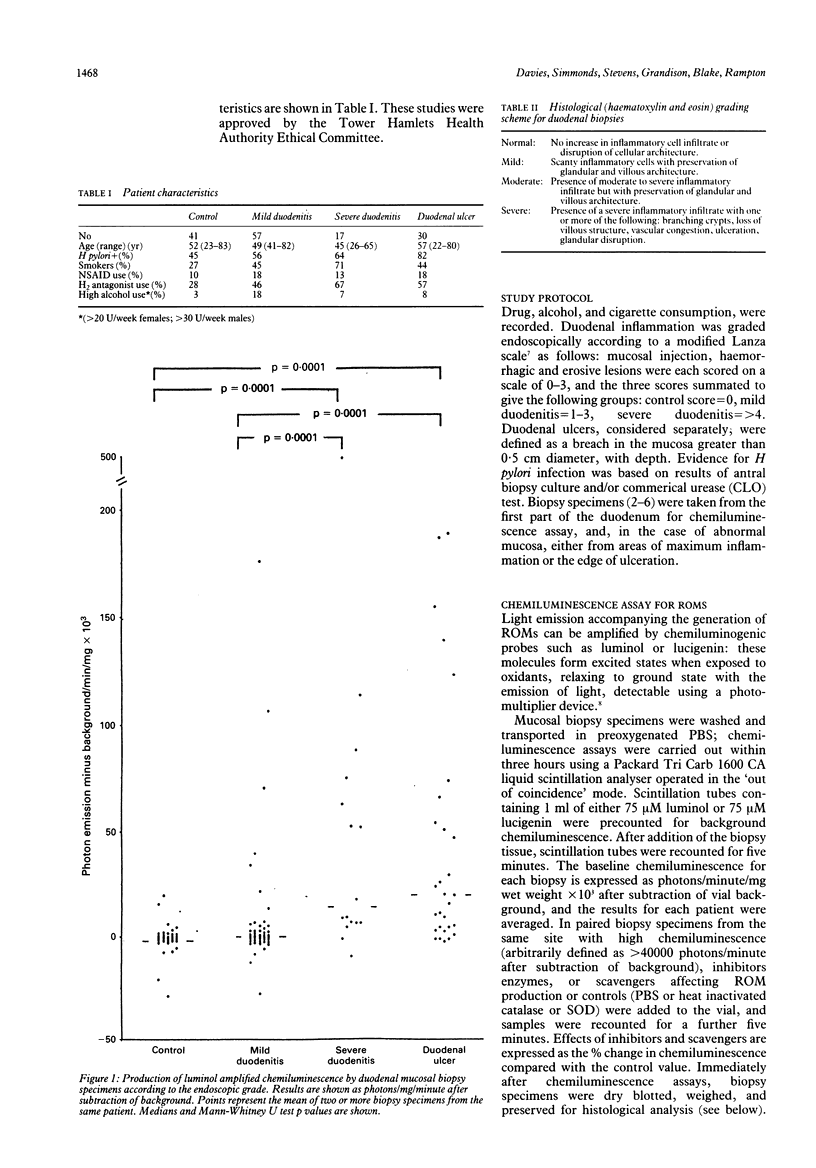
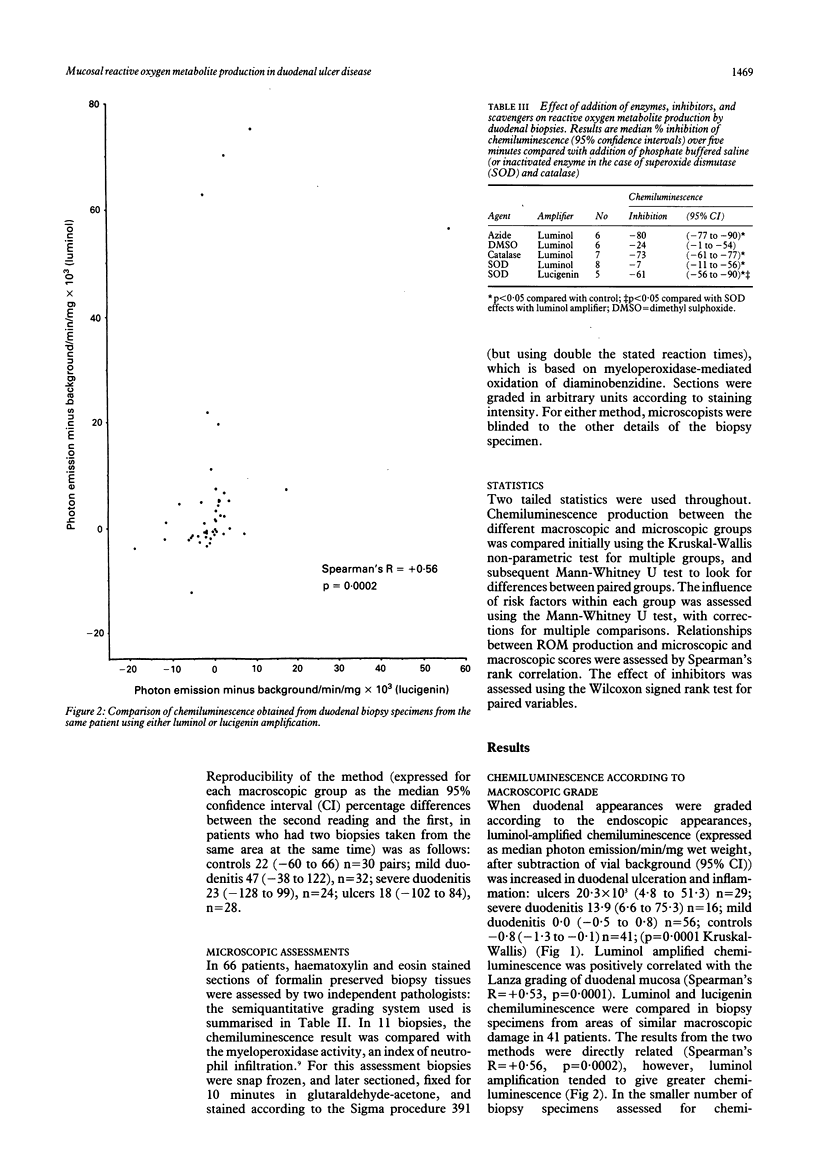
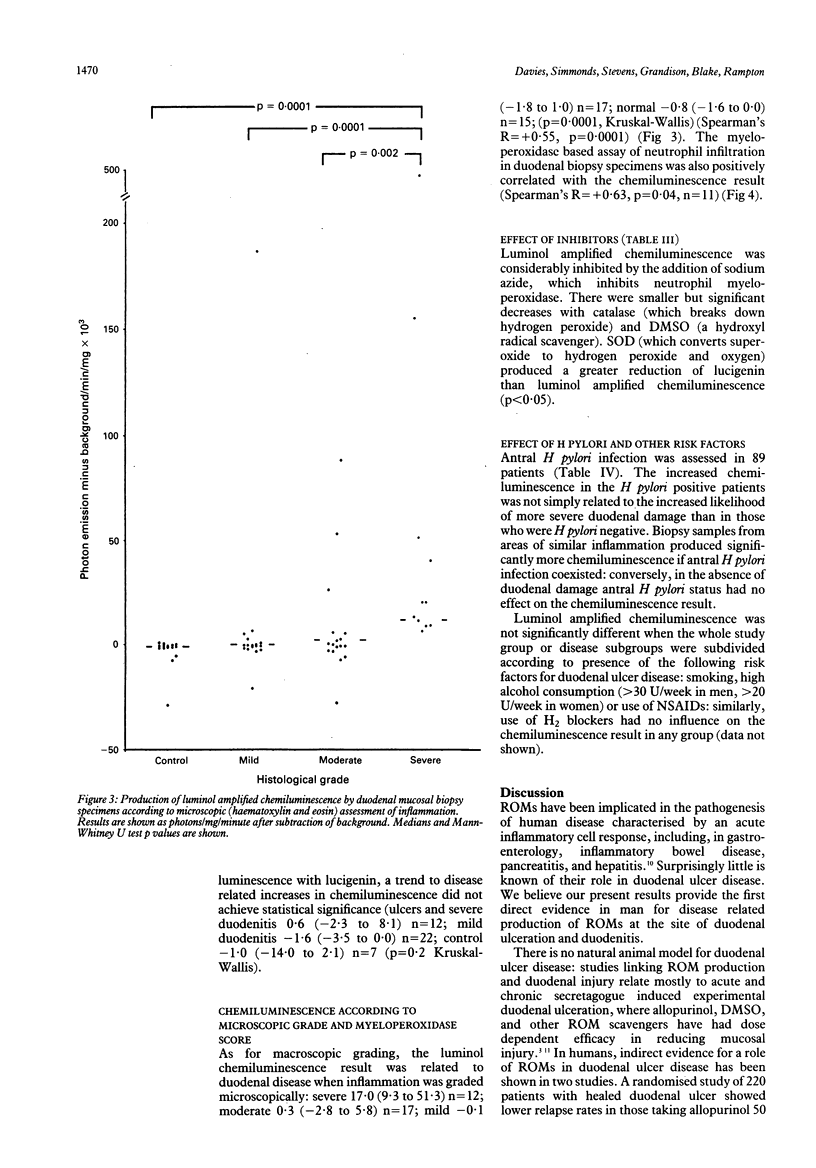
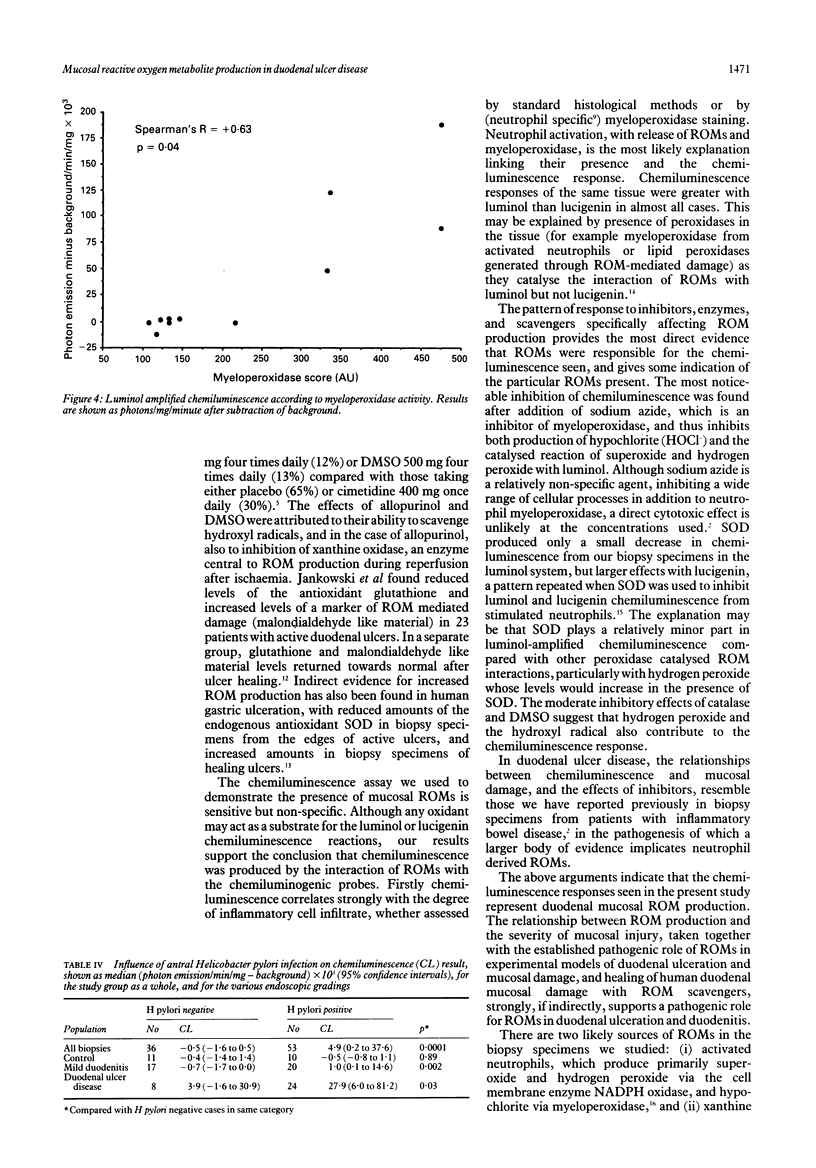
To investigate the hypothesis that reactive oxygen metabolites are important in the pathophysiology of duodenal ulcer disease, their production by duodenal mucosal biopsy specimens was measured using luminol and lucigenin amplified chemiluminescence. Luminol chemiluminescence, expressed as background corrected median photon emission/mg/min x 10(3) (95% confidence intervals), was increased in duodenal inflammation as assessed macroscopically: ulcers 20.3 (4.8 to 51.3), n = 29; severe duodenitis 13.9 (6.6 to 75.3), n = 16; mild duodenitis 0.0 (-0.5 to 0.8), n = 56; controls -0.8 (-1.3 to -0.1), n = 41; p = 0.0001, Kruskal-Wallis) and microscopically: severe 17.0 (9.3 to 51.3), n = 12; moderate 0.3 (-2.8 to 5.8), n = 17; mild -0.1 (-1.8 to 1.0), n = 17; controls -0.8 (-1.6 to 0.0), n = 15; (p = 0.0001). Luminol chemiluminescence was directly related to both the macroscopic and microscopic severity of duodenal damage (Spearman's R = + 0.53, + 0.55 respectively, both p = 0.0001), to histochemical assessment (myeloperoxidase activity) of neutrophil infiltration (R = + 0.63; p = 0.04), and to lucigenin chemiluminescence (R = + 0.56, p = 0.0002). Luminol chemiluminescence was inhibited by sodium azide (-80%), catalase (-73%), and dimethyl sulphoxide (-24%). Superoxide dismutase inhibited lucigenin more than luminol dependent chemiluminescence (-61% and -7% respectively, p < 0.05). Within disease groups, Helicobacter pylori antral infection was associated with increased duodenal chemiluminescence, whereas smoking, alcohol, and use of NSAIDs or H2 blockers had no influence. Their disease related generation in duodenal mucosa supports a role for reactive oxygen metabolites in the pathogenesis of duodenitis and duodenal ulcer. These metabolites might include superoxide, hydrogen peroxide, hydroxyl, and products of myeloperoxidase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 1986;133:449–493. doi: 10.1016/0076-6879(86)33085-4. [DOI] [PubMed] [Google Scholar]
- Chakraborti S., Gurtner G. H., Michael J. R. Oxidant-mediated activation of phospholipase A2 in pulmonary endothelium. Am J Physiol. 1989 Dec;257(6 Pt 1):L430–L437. doi: 10.1152/ajplung.1989.257.6.L430. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Twohig B., Arfors K. E., Harlan J. M., Granger D. N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Krawisz J. E., Sharon P., Stenson W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984 Dec;87(6):1344–1350. [PubMed] [Google Scholar]
- Lanza F. L. Endoscopic studies of gastric and duodenal injury after the use of ibuprofen, aspirin, and other nonsteroidal anti-inflammatory agents. Am J Med. 1984 Jul 13;77(1A):19–24. doi: 10.1016/s0002-9343(84)80014-5. [DOI] [PubMed] [Google Scholar]
- Lewis M. S., Whatley R. E., Cain P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J Clin Invest. 1988 Dec;82(6):2045–2055. doi: 10.1172/JCI113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney C., Keenan J., Munster D., Wilson I., Allardyce R., Bagshaw P., Chapman B., Chadwick V. Neutrophil activation by Helicobacter pylori. Gut. 1991 Aug;32(8):853–857. doi: 10.1136/gut.32.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Salim A. S. Oxygen-derived free radicals and the prevention of duodenal ulcer relapse: a new approach. Am J Med Sci. 1990 Jul;300(1):1–8. doi: 10.1097/00000441-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Salim A. S. Role of oxygen-derived free radicals in mechanism of acute and chronic duodenal ulceration in the rat. Dig Dis Sci. 1990 Jan;35(1):73–79. doi: 10.1007/BF01537226. [DOI] [PubMed] [Google Scholar]
- Salim A. S. Role of oxygen-derived free radicals in the mechanism of chronic gastric ulceration in the rat: implications for cytoprotection. Digestion. 1989;43(1-2):113–119. doi: 10.1159/000199868. [DOI] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds N. J., Allen R. E., Stevens T. R., Van Someren R. N., Blake D. R., Rampton D. S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992 Jul;103(1):186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- Takamasu M., Fuse Y., Kawamoto K., Kodama T., Ohishi T. Possible mechanisms of diethyldithiocarbamate-induced gastro-duodenal mucosal damage in rats. Scand J Gastroenterol Suppl. 1989;162:112–115. doi: 10.3109/00365528909091138. [DOI] [PubMed] [Google Scholar]



