Abstract
Cold-inducible RNA-binding protein (CIRP) was originally found in mammalian cells as a protein that is overexpressed upon a temperature downshift. Recently, we identified a Xenopus homolog of CIRP, termed xCIRP2, as a major cytoplasmic RNA-binding protein in oocytes. In this study we found by yeast two-hybrid screening that the Xenopus homolog of protein arginine N-methyltransferase 1 (xPRMT1) interacted with xCIRP2. We found that an arginine- and glycine-rich region of xCIRP2, termed the RG4 domain, was a target of xPRMT1 for methylation in vitro. xCIRP2 expressed in cultured cells accumulated in the nucleus as does mammalian CIRP. Interestingly, the RG4 domain was necessary for nuclear localization of xCIRP2. RG4-mediated nuclear accumulation of xCIRP2 was diminished in the presence of transcription inhibitors, suggesting that nuclear localization of xCIRP2 was dependent on ongoing transcription with RNA polymerase II. Analysis of interspecies heterokaryons revealed that xCIRP2 was capable of nucleocytoplasmic shuttling and the RG4 domain functioned as a nucleocytoplasmic shuttling signal. Methylation by overexpressed xPRMT1 caused cytoplasmic accumulation of xCIRP2. Possible implications of the relationship between regulation of intracellular localization and multiple functions of xCIRP2 will be discussed.
INTRODUCTION
In eukaryotic cells, gene expression is regulated post-transcriptionally as well as transcriptionally, and many RNA-binding proteins play pivotal roles in mRNA metabolism, such as pre-mRNA processing, mRNA export, translation and mRNA turnover (1–5). In the nucleus, more than 20 species of hnRNP proteins package pre-mRNA into complexes called hnRNPs (heterogeneous nuclear ribonucleoprotein particles) (6). A subset of hnRNP proteins is restricted to the nucleus, while others continuously shuttle between the nucleus and cytoplasm (3,7). Previous studies on the transport of hnRNP A1 protein identified a nucleocytoplasmic shuttling signal (NSS), the M9 domain, that functions as both a nuclear localization signal and a nuclear export signal (8–10). Later on, shuttling signals have been identified in RNA-binding proteins such as hnRNP K, HuR and SR proteins (11–15). Nuclear import receptors for some of these NSS have been identified, yet export receptors are unknown (13,16,17). These nucleocytoplasmic shuttling proteins can exit the nucleus accompanying mRNA and affects the cytoplasmic fate of mRNA.
Cold-inducible RNA-binding protein (CIRP) was initially identified from the mouse testis by cDNA cloning of an RNA-recognition-motif (RRM)-containing RNA-binding protein (18). CIRP consists of an N-terminal RRM and a C-terminal flanking glycine-rich region containing arginine-glycine-glycine (RGG) repeats. This modular structure is similar to those of hnRNP A/B proteins, represented by hnRNP A1, except that hnRNP A/B proteins have two N-terminal RRMs. Mouse CIRP is highly expressed in the testis within the scrotum, which is maintained at temperatures lower than other parts of the body cavity, while its expression is repressed by exposing the testis to heat stress (19). The expression level of CIRP in cultured mouse cells increases upon a temperature downshift from 37 to 32°C and CIRP mediates the cold-induced cell growth suppression presumably by prolonging the G1 phase of the cell cycle, although the underlying mechanisms are still poorly understood (18).
CIRP homologs from human, rat, Mexican axolotl, bull frog and Xenopus laevis were identified (20–24). To date three Xenopus CIRP homologs were identified: XCIRP, XCIRP-1 and xCIRP2 (23,25,26). XCIRP protein consists of 163 amino acids showing 74% identity to mouse CIRP and its mRNA is highly expressed at a particular stage of pronephros formation at an early developmental stage (23). XCIRP-1 was cloned as a major isoform of Xenopus CIRP, which differs from XCIRP in the 3′ untranslated region (UTR) (26). Microinjection of antisense RNA into Xenopus embryos produces tailbuds with deformations of the brain and internal organs. Depletion of maternal XCIRP-1 mRNA also disrupts the morphogenetic migration of the blastomeres in pronephros lineage. We reported another Xenopus CIRP homolog, xCIRP2, as a major cytoplasmic RNA-binding protein in oocytes (25). xCIRP2 protein consists of 166 amino acids and shows >90% identity to XCIRP and XCIRP-1. xCIRP2 3′UTR is highly homologous to that of XCIRP-1 and the temporal expression patterns of the xCIRP2 mRNA during Xenopus early development is similar to XCIRP-1 mRNA, suggesting that xCIRP2 and XCIRP-1 represent two allelic forms. xCIRP2 mRNA and protein are highly expressed in oocytes, and in an adult frog xCIRP2 protein is most abundant in ovary, testis and brain. In a previous study, we examined the RNA-binding activity of xCIRP2 and demonstrated its cytoplasmic localization in the oocyte and possible association with ribosomes (25). Taken together, it has been clarified that Xenopus CIRP plays key roles in differentiation and morphogenesis during early development. However, the molecular mechanisms by which Xenopus CIRP regulates RNA metabolism and thereby affects the embryonic development are still elusive.
Recently, there has been a magnified interest in the regulation of protein function by arginine methylation (27). Various hnRNP proteins, including hnRNP A1, were reported to be methylated in vivo (28–30). A variety of protein substrates are methylated on arginine residues by protein-arginine methyltransferase 1 (PRMT1), a mammalian predominant type I arginine methyltransferase that catalyzes the asymmetric dimethylation of arginine residues (31–35). Previous studies on the substrate specificity of arginine methylation in hnRNP A1 and other RNA-binding proteins identified a preferable recognition motif of (F/G)GGRG G(G/F) (36). This sequence includes the RGG domain found in many RNA-binding proteins (30,36). The impact of this modification on in vivo function of hnRNP proteins is largely unclear.
In this report, we describe the identification of a Xenopus homolog of PRMT1 as an xCIRP2-binding protein. We examined the subcellular localization of xCIRP2 and identified an NSS containing RGG repeats in xCIRP2, which directed bidirectional trafficking of fusion proteins in cultured cells. Furthermore, we found that methylation of xCIRP2 by xPRMT1 resulted in the accumulation of xCIRP2 in the cytoplasm. Our results suggested that xCIRP2 and possibly mammalian CIRP serve to link RNA metabolism in the nucleus and the cytoplasm.
MATERIALS AND METHODS
Nucleotide sequence accession number
The complete nucleotide sequence of xPRMT1 cDNA obtained in this study will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession no. AB085173.
Yeast two-hybrid screening
The xCIRP2-coding region was amplified by polymerase chain reaction (PCR) using a primer set of 5′-CGCGAATTCATGTCTGATGAAGGAAAAC-3′ and 5′-AGACGCGTCGACCTCGTGTGTAGCATAAC-3′ with the xCIRP2 cDNA as the template (25). This fragment was digested with EcoRI and SalI and subcloned into the GAL4 DNA-binding domain fusion vector pGBT9 (Clontech) to create a ‘bait’ plasmid pGBT9-xCIRP2. A X.laevis oocyte MATCHMAKER cDNA library in the GAL4 activation domain vector pACT2 (Clontech) was used as ‘prey’ plasmids for screening.
Yeast two-hybrid screening to identify proteins that interact with xCIRP2 was performed according to the manufacturer’s instructions. Briefly, the yeast strain AH109 was transformed to a leucine prototrophic strain using pGBT9-xCIRP2. The strain was then transformed with the cDNA library. In total, 1 × 107 transformants were plated on the Synthetic Dropout (SD) medium lacking adenine, histidine, leucine and tryptophan to select for interacting clones. Viable colonies were assayed for α-galactosidase activity by plating on an ade-his-leu-trp-free SD medium containing 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside. Thirty-nine cDNA clones, which positively interacted with xCIRP2, were isolated and sequenced using an Applied Biosystems model 377 DNA sequencer.
Screening of a cDNA clone containing the entire open reading frame of Xenopus PRMT1
A 483-bp fragment based on the sequence of the EST clone dab88b08.y1 (GenBank accession no. BG359836) was amplified by PCR from a Xenopus oocyte total cDNA using the primers 5′-ATGGAGAACTTTGTAGCCAAGTTGGCC-3′ and 5′-CCATTCACTGATTATGATGTCC-3′ and was used as a probe to screen a Xenopus oocyte cDNA library as described previously (37).
Preparation of recombinant proteins
To obtain the glutathione S-transferase (GST) fusion construct with xCIRP2, xCIRP2 cDNA was used for PCR amplification with a primer set, 5′-CCGCGAATTCCATGTCTGATGAAGGAAAAC-3′ and 5′-AGCCCGCTCGAGCTCGTGTGTAGCATAAC-3′. To obtain GST fusion construct with the RG4 domain, xCIRP2 cDNA was used for PCR amplification with a primer set, 5′-CCGGAATTCCAGAAGAGGTGGTTACAGAGGTGGC-3′ and 5′-CCGCTCGAGTCAACCGCCATAACCTCCACTTCTG-3′. The PCR products were digested with EcoRI and XhoI and the resulting fragments were then cloned into the EcoRI- and XhoI-digested pGEX 4T-3 vector (Amersham Bioscience, Inc.).
To obtain the N-terminal hemagglutinin (HA) and C-terminal 6× histidine-tagged proteins, HA-xCIRP2 and HA-xCIRP2ΔRG4, the xCIRP2 cDNA and the GFPΔRG4 construct (see below) were used for PCR amplification with a primer set, 5′-GGCAGCCATATGTACCCATACGACGTCCCAGACTACGCTTCTGATGAAGGAAAACTCTTTATC-3′ and 5′-AGCCCGCTCGAGCTCGTGTGTAGCATAAC-3′. The PCR products were digested with EcoRI and PstI and the resulting fragments were then cloned into the pET-24b vector (Novagen). To obtain the 6× histidine-tagged construct for the xPRMT1 protein, xPRMT1 cDNA was used for PCR amplification with a primer set, 5′-ATGCGTCGACATGGCCGAAGCGACCACCTGC-3′ and 5′-CCGCTCGAGACGCATTCTGTAGTCTGTTGAAC-3′. The PCR product was digested with EcoRI and XhoI and the resulting fragment was then cloned into the pET-24b vector. To obtain recombinant proteins, Escherichia coli BL21 (DE3) cells were transformed with the appropriate construct. Overexpression and purification of recombinant proteins were performed as described previously (25,37).
GST pull-down assay
In order to examine the interaction between xCIRP2 and xPRMT1 in vitro, 1 µg GST or GST-xCIRP2 was incubated with 2 µg of 6× histidine-tagged xPRMT1 in 100 µl of reaction mixture containing 20 mM Tris–HCl (pH 7.5) and 100 mM NaCl with or without 80 µM S-adenosyl-l-methionine (AdoMet; Nacalai Tesque, Kyoto, Japan) at 37°C for 60 min. Glutathione–Sepharose beads were then added into the reaction mixture and the mixture was incubated at 4°C for 60 min, following which the beads were washed four times in wash buffer [20 mM Tris–HCl (pH 7.5), 100 mM NaCl, 1 mM dithiothreitol (DTT) and 0.5 mM phenylmethanesulfonyl fluoride (PMSF)]. After removing the final wash, the samples were analyzed by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and the gel was stained with silver.
In vitro methylation of xCIRP2
In a standard methylation reaction, 1 µg of recombinant xCIRP2 and 0.5 µg of xPRMT1 were incubated in the presence of 25 pmol of S-adenosyl-l [methyl-3H] methionine ([3H] AdoMet; 79 Ci/mmol; Amersham Biosciences, Inc.) in 20 μl of 25 mM Tris–HCl (pH 7.5) at 37°C for 60 min (38). After the reaction, proteins were analyzed by SDS–PAGE and the gel was subjected to fluorography.
Cell culture, transfection and immunofluorescence
To obtain the eukaryotic expression vectors that express xCIRP2 with the C-terminal HA-tag and its N- or C-terminal deletion mutants fused to green fluorescent protein (GFP), DNA fragments derived from the xCIRP2-coding region were generated using PCR amplification and cloned into the pEGFP-C1 vector (Clontech). To obtain the GFP-xCIRP2ΔRG4 expression vector, the xCIRP2 cDNA was used for PCR amplification with a primer set, 5′-CCGCGAATTCCATGTCTGATGAAGGAAAAC-3′ and 5′-CCGCGAATTCGCATCACCAGAAGAATTGCC-3′. The PCR pro duct was digested with EcoRI and the resulting fragment was then cloned into EcoRI-digested pEGFP-C1/C3, which contains a DNA fragment encoding the xCIRP2-C3 mutant (see Fig. 3B) in the EcoRI and PstI sites of pEGFP-C1. The RG4 domain of human CIRP from HeLa total RNA was cloned by PCR. To construct eukaryotic expression vector that expresses c-myc epitope-tagged xPRMT1, xPRMT1 cDNA was used for PCR amplification with a primer set, 5′-TTACTACGCGTGAGAGATGGCCGAAGCGACCACCTGC-3′ and 5′-TGCTCTAGAGTCGACTCACAGATCCTCTTCTGAGATGAGTTTTTGTTCACGCATTCTGTAGTCTGTTGAAC-3′. The PCR product was digested with MluI and SalI and the resulting fragment was then cloned into the MluI- and SalI-digested pCIneo vector (Promega). The xPRMT1 fragment was then isolated from the plasmid by digesting with SalI and XhoI and cloned into XhoI-digested pCAGGS (a kind gift from Dr J. Miyazaki) (39).
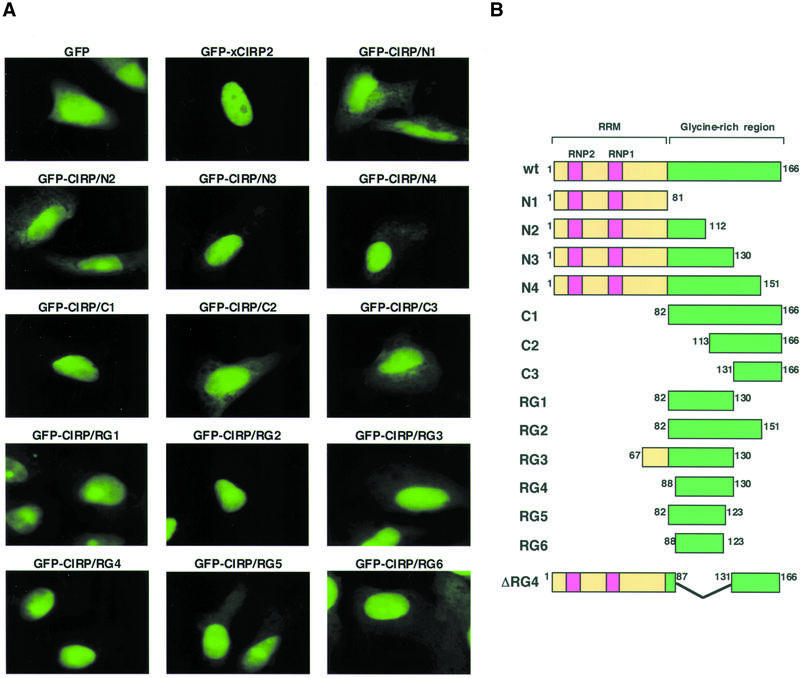
Figure 3.
Identification of the domain, which functions as a nuclear localization signal. (A) Fluorescent micrographs showing subcellular localization of GFP and the GFP-xCIRP2 derivatives. HeLa S3 cells were transfected with plasmid DNA to express GFP fusion xCIRP2 derivatives. The localization of the GFP fusion proteins was examined under a fluorescence microscope. (B) Schematic diagrams of xCIRP2 protein and its deletion mutants expressed in HeLa S3 cells. GFP is fused at the N-terminus of xCIRP2.
HeLa S3 cells were grown at 37°C in Dulbecco’s modified Eagle’s medium supplemented with 10% calf serum. Cells grown on a glass coverslip in a 24-well plate were transfected with 200 ng of DNA using the Effectene transfection reagent (Qiagen), and cultured at 37°C for 48 h. For treatment with transcriptional inhibitors, the cells were incubated in the medium containing either 10 µg/ml actinomycin D or 0.1 mM 5,6-dichlororibofuranosyl benzimidazole (DRB) for 4 h. DRB was then removed by changing the medium to fresh medium and incubating further for 2 h prior to fixation. For treatment with a methyltransferase inhibitor, the cells were incubated in the medium containing 20 µM adenosine dialdehyde (AdOx) for 24 h prior to transfection and AdOx was included in the culture medium until cell fixation. The cells were washed three times with phosphate-buffered saline (PBS) and then fixed with PBS containing 4% paraformaldehyde at room temperature for 30 min. The fixed cells were washed with PBS containing 0.3% Triton X-100 and then blocked with 2.5% bovine serum albumin (BSA) in PBS. After blocking, the cells were incubated for 1 h with 10 µg/ml anti-c-myc antibody clone 9E10 (Roche) in PBS containing 2.5% BSA. The cells were then washed three times with PBS containing 0.3% Triton X-100 and incubated with a secondary antibody, Alexa Fluor 488-conjugated anti-mouse IgG (Molecular Probes). After 1 h, the cells were washed three times with PBS containing 0.3% Triton X-100, mounted on a glass slide with the PermaFluor aqueous mounting medium (Immunon) and examined under a fluorescence microscope.
Heterokaryon analysis
On day 1, subconfluent HeLa cells were transfected with the expression vectors encoding GFP-xCIRP2 or GFP-RG4. Twenty-four hours later the cells were trypsinized and transferred to a 24-well plate containing glass coverslips. The cells were split such that they would be at 40% confluence on the morning of day 3 of the experiment. At this time, an equal number of NIH 3T3 cells were seeded into the wells. The co-cultures were then incubated at 37°C for another 4 h to allow the 3T3 cells to attach to glass coverslips. The medium containing 100 µg/ml cycloheximide was then added and the co-cultures were incubated for another 30 min to inhibit protein synthesis. The coverslips were rinsed with PBS and the cells were fused by inverting the coverslip onto 50 µl of 50% polyethyleneglycol 4000 for 120 s. The coverslips were then rinsed with PBS and returned to a fresh medium containing 100 µg/ml cycloheximide for another 60 min and then incubated in PBS containing 5 µg/ml Hoechst 33342 for 5 min prior to fixation (14).
Subcellular fractionation
To obtain cytoplasmic and nuclear fractions, HeLa S3 cells were washed with PBS, scraped, incubated in hypotonic buffer [5 mM Tris–HCl (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT and 0.25 mM PMSF] on ice for 10 min and passaged three times through a 27 gauge needle. Nuclei were pelleted by centrifugation at 3500 r.p.m. for 5 min and the supernatants were pooled as cytoplasmic fractions. For preparing nuclear extracts, nuclei were resuspended in hypotonic buffer, added an equal volume of extraction buffer [15 mM Tris–HCl (pH 7.5), 0.8 M NaCl, 1.5 mM MgCl2, 1 mM DTT and 0.25 mM PMSF] and incubated on ice for 30 min. The samples were centrifuged as above and the supernatants were then pooled as nuclear extracts. The nuclear pellets were re-suspended in PBS and sonicated. Further fractionation of the cytoplasmic fraction by ultracentrifugation was performed as described previously (25). Each fraction was analyzed by SDS–PAGE and subjected to immunoblotting with anti-HA antibody (clone 3F10, Roche).
RESULTS
Identification of xPRMT1 as an xCIRP2-interacting protein
As a step exploring cellular functions of xCIRP2, we searched for proteins that interact with xCIRP2 by yeast two-hybrid screening. A yeast strain AH109 expressing the full-length xCIRP2 fused to the GAL4 DNA-binding domain as the bait was transformed with a cDNA library in which the GAL4 activation domain is fused to cDNAs from Xenopus oocytes. Based on the ability of positive clones to grow on media lacking adenine and histidine and to express α-galactosidase, 39 cDNA clones were isolated and subjected to sequence analysis. A BLAST search of protein sequence database revealed that the predicted open reading frame encoded by one of the positive clones, clone 4.32, shows significant homology to mammalian PRMT1 (Fig. 1). Sequence analysis of clone 4.32 revealed that it was an incomplete clone, lacking the 5′ region encoding the N-terminal end of the protein. By using the sequence information of an EST clone dab88b08.y1 that corresponds to the 5′ region of clone 4.32, a full-length cDNA was cloned from a Xenopus cDNA library. This cDNA encoded an open reading frame of 369 amino acids. The primary structural comparison of the predicted open reading frame encoded by the cDNA revealed high homology with human PRMT1 (86%) (40), Caenorhabditis elegans NM_075508 (60%), Drosophila melanogaster CG6554 (62%), Arabidopsis thaliana pam1 (54%) and Saccharomyces cerevisiae Hmt1p/Rmt1p (45%) (41). We, therefore, termed this protein encoded by the full-length clone 4.32 cDNA Xenopus protein-arginine methyltransferase 1 or xPRMT1.
Figure 1.
xPRMT1 interacts with xCIRP2. (A) Interaction of xCIRP2 and clone 4.32 in yeast two-hybrid analysis. AH109 yeast cells were transformed with each plasmid and spread on the minimal SD medium lacking adenine, histidine, leucine and tryptophan. (B) cDNA cloning of xPRMT1. The deduced amino acid sequence of cloned xPRMT1 is aligned with previously reported amino acid sequences, namely, human PRMT1 (accession no. CAA71764) (40), C.elegans NM_075508 (NP_507909.1), D.melanogaster CG6554 (XP_082348), A.thaliana pam1 (AAL36326) and S.cerevisiae Hmt1p/Rmt1p (NP_009590) (41,65,66). Amino acids identical in all six sequences are indicated by asterisks.
To determine whether xPRMT1 interacts with xCIRP2 in vitro, the recombinant xCIRP2 and xPRMT1 proteins were prepared in E.coli as described in the Materials and Methods. In a GST pull-down assay, xPRMT1 specifically interacted with GST-xCIRP2 but not with GST (Fig. 2A). The interaction between xCIRP2 and xPRMT1 was observed irrespective of the presence of the methyl group-donor, AdoMet.
Figure 2.
In vitro arginine methylation of xCIRP2 protein by recombinant xPRMT1. (A) Physical interaction of xCIRP2 and xPRMT1 was analyzed by GST pull-down assay. One microgram of GST (lanes 1 and 2) or GST-xCIRP2 (lanes 3 and 4) was incubated with 2 µg of xPRMT1 in 100 µl of 20 mM Tris–HCl (pH 7.5), 100 mM NaCl without (lanes 1 and 3) or with (lanes 2 and 4) 80 µM AdoMet. Half of each reaction was analyzed by SDS–PAGE and the gel was stained with silver. One hundred nanograms of GST, GST-xCIRP2 and xPRMT1 proteins were electrophoresed in lanes 5, 6 and 7, respectively. (B) One microgram of recombinant HA-xCIRP2 protein (lanes 2, 4, 6 and 8) and 0.5 µg of xPRMT1 protein (lanes 3, 4, 7 and 8) were incubated in 20 µl of 20 mM Tris–HCl (pH 7.5) containing [3H]AdoMet. Proteins were separated by SDS–PAGE and stained with Coomassie Brilliant Blue (CBB staining). The gel was then dried and subjected to fluorography (Methylation). (C) One microgram of recombinant HA-xCIRP2 (lanes 1 and 5), HA-xCIRP2ΔRG4 (lanes 2 and 6), GST (lanes 3 and 7) and GST-RG4 (lanes 4 and 8) were methylated and visualized as in (B). (D) Schematic diagrams of recombinant xCIRP2 protein and its mutants used for in vitro methylation analysis. RNP1 and RNP2 are the conserved sequences in RRM (67).
xCIRP2 is methylated by xPRMT1 in vitro
We next examined whether xCIRP2 is methylated by xPRMT1. The recombinant substrates are probably not methylated in E.coli, which lacks protein arginine methyltransferase activity (30). For in vitro methylation, the recombinant xCIRP2 was incubated with xPRMT1 in the presence of 3H-labeled AdoMet at 37°C for 60 min. Under these conditions, the xCIRP2 protein was methylated by xPRMT1 (Fig. 2B).
Earlier studies on partially purified hnRNP particles showed that hnRNP complexes contained an amino acid, NG,NG-dimethylarginine (28,30). In many cases, methylation appears to occur at an arginine residue in an RGG motif. In hnRNP A1, for instance, four arginine residues are dimethylated in vivo, three of which are localized in the context of RGG sequences (36,42). We therefore reasoned that xPRMT1 might methylate the C-terminal glycine- and arginine-rich region of xCIRP2 that contains four RGG repeats. To test this, we generated an xCIRP2 deletion mutant (xCIRP2ΔRG4) that lacks an arginine- and glycine-rich region (Arg88–Gly130) termed the RG4 domain, which contains four RGG motifs and, as the complement, the RG4 domain fused to GST (GST-RG4) was also prepared (Fig. 2D). Methylation analysis of these proteins by xPRMT1 revealed that the full-length xCIRP2 and GST-RG4 were methylated, whereas xCIRP2ΔRG4 and GST showed no detectable methylation (Fig. 2C). As shown in Figure 2B lanes 7 and 8, xPRMT1 was weakly labeled with [3H]AdoMet. This may have resulted from a self-methylation by xPRMT1 or residual binding of [3H]AdoMet in its methyl group-donor-binding site observed even after the analysis by SDS–PAGE. Nevertheless, these results clearly showed that the RG4 domain of xCIRP2 was a PRMT1 substrate for arginine methylation.
xCIRP2 localizes in oocyte cytoplasm and nuclei of cultured cells
In our previous report we observed the cytoplasmic localization of xCIRP2 in Xenopus oocytes (25). In contrast, mouse CIRP that is induced in BALB 3T3 cells by a temperature downshift from 37 to 32°C localizes in the nuclei (18). We then examined the localization of xCIRP2 in somatic cells. The expression vector of GFP fused to xCIRP2 was transfected into human HeLa S3 cells and localization of the fusion proteins was examined under a fluorescence microscope (Fig. 3). As shown in Figure 3A, while GFP itself was distributed in both the cytoplasm and nuclei, with a slightly higher signal in nuclei, GFP-xCIRP2 exclusively localized in the nuclei of HeLa S3 cells and several other cultured cells (data not shown), consistent with the previous results for mouse CIRP in BALB 3T3 cells (18). Our observations, along with results of these studies, suggest that xCIRP2 shows cytoplasmic localization in the oocytes despite its potential to localize in the nucleus.
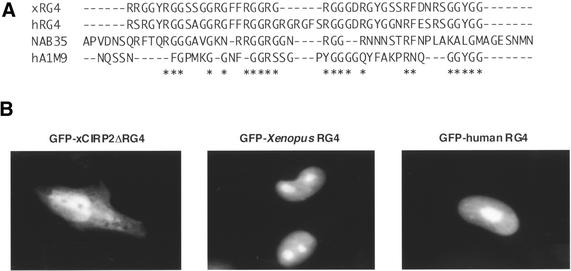
To determine the domains of xCIRP2 that direct the protein to localize in the nuclei of cultured cells, mammalian expression vectors of GFP fused to xCIRP2 deletion mutants were prepared and transfected into HeLa S3 cells as schematically drawn in Figure 3B. Our domain analysis revealed that the N3, N4, C1, RG1, RG2 and RG4 mutants also showed exclusive nuclear accumulation. However, RG3, RG5 and RG6 localized to the nucleus at reduced concentration and N1, N2, C2 and C3 mutants did not localize to the nucleus. All the mutants that exclusively accumulated in the nucleus contain 43 amino acid residues consisting of typical glycine-rich sequences, RGG repeats and GGYGG (Fig. 4A). Strikingly, this region corresponds to the RG4 domain that could be methylated with xPRMT1 (Fig. 2). So far several arginine- and glycine (RG)-rich type nuclear localization signals have been identified in hnRNP or hnRNP-like proteins. The M9 of hnRNP A1 and the NAB35 of yeast Nab2p poly(A)+ mRNA-binding protein were compared with the xCIRP2 RG4 sequence and the corresponding region in human CIRP in Figure 4A (8,21,43). To assess the significance of this sequence, a GFP-xCIRP2 deletion mutant, ΔRG4, was transfected into HeLa cells. The GFP-xCIRP2ΔRG4 did not accumulate in the nucleus while the GFP-Xenopus RG4 domain localized to the nucleus (Fig. 4B). We therefore concluded that the nuclear localization signal of xCIRP2 resided in the carboxyl part of the protein corresponding to the RG4 domain. The domain is conserved in other CIRP homologs and we confirmed that the RG4 domain of human CIRP, which contains three RGG repeats, also accumulated in the nuclei of HeLa cells (Fig. 4A and B). Interestingly, our xCIRP2 deletion mutants, which retain RG4, accumulated in the nucleoli in contrast to nucleolar exclusion of GFP-xCIRP2. GFP-RG4 probably diffused into the nucleolus and were retained by non-specific binding of the RGG domain to ribosomal RNA or ribosomal proteins, as speculated for the RS domain of U2AF (44,45).
Figure 4.
RG-rich domains function as nuclear localization signals. (A) Sequence alignment of RG-rich domains function as NLSs. Sequences shown correspond to amino acids 88–130 in xCIRP2 (25), 89–137 in human CIRP (21), 197–252 in yeast Nab2p (NAB35) (43) and 267–291 in human hnRNP A1 (M9) (8). Amino acids that are identical in at least three NLSs are indicated by asterisks. (B) Fluorescent micrographs show subcellular localization of the GFP-xCIRP2ΔRG4, GFP-Xenopus RG4 and GFP-human RG4. Schematic diagrams of the GFP-xCIRP2ΔRG4 and GFP-Xenopus RG4 are shown in Figure 3B.
xCIRP2 is a nucleocytoplasmic shuttling protein dependent on RG4 domain
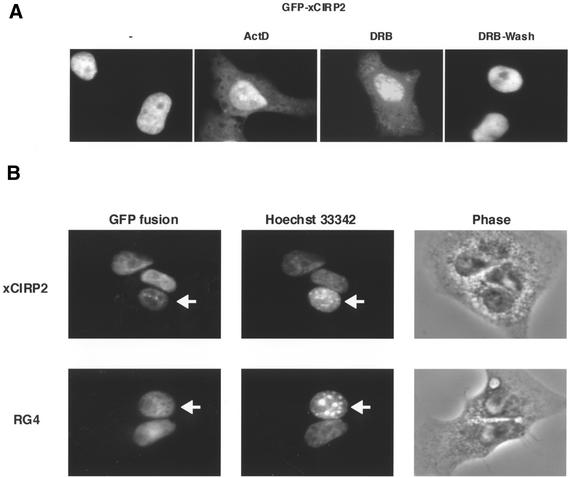
We next explored the conditions under which exogenously expressed xCIRP2 accumulates in the cytoplasm of somatic cells. In a previous study, hnRNP A1 was shown to accumulate in the cytoplasm of cells treated with transcriptional inhibitors such as actinomycin D or DRB (46). Actinomycin D and DRB are RNA polymerase II inhibitors but their mechanisms of inhibition differ from each other (47). The modular structure of xCIRP2 is similar to that of hnRNP A1, which consists of two RRMs and a flanking glycine-rich region containing RGG repeats. Accordingly, HeLa cells transfected with the GFP-xCIRP2 expression vector were treated with transcriptional inhibitors. After treatment with 10 µg/ml actinomycin D or 0.1 mM DRB for 4 h, significant part of GFP-xCIRP2 accumulated in the cytoplasm as compared with the control without the transcriptional inhibitors (Fig. 5A). Removal of the reversible transcriptional inhibitor, DRB, resulted in the nuclear accumulation of xCIRP2. The presence of a protein synthesis inhibitor, cycloheximide, during the course of the experiments did not further affect the localization of the xCIRP2 protein (data not shown, also see Fig. 5B). This suggests that xCIRP2 found in the cytoplasm after treatment with the transcriptional inhibitors was pre-existing xCIRP2 that was exported from the nucleus. Taken together, these results indicate that xCIRP2 is able to shuttle between the nucleus and the cytoplasm, dependent on ongoing transcription in the nucleus as is hnRNP A1.
Figure 5.
Nucleocytoplasmic shuttling of xCIRP2. (A) xCIRP2 accumulates in the cytoplasm of cultured cells by treatment with transcriptional inhibitors. The GFP-xCIRP2 expression vector was transfected into HeLa S3 cells. The cells were treated with 10 µg/ml actinomycin D or 0.1 mM DRB at 37°C for 4 h, and then DRB was removed by changing the medium with fresh medium. The culture was further incubated at 37°C for 2 h. The subcellular localization of the GFP fusion proteins was analyzed by fluorescence microscopy. (B) xCIRP2 and the RG4 domain shuttle between nucleus and cytoplasm. Expression vectors of GFP-xCIRP2 (upper panels) and GFP-xRG4 (lower panels) were transfected into HeLa cells. Two days later, the cells were fused with NIH 3T3 cells to form heterokaryons and incubated in the medium containing 100 µg/ml of cycloheximide for 1 h. Heterokaryons formed between HeLa cells (human) and NIH 3T3 cells (mouse) were examined by florescence microscopy of GFP fusion proteins and nuclei stained with Hoechst 33342. The arrows indicate murine NIH 3T3 cells. ‘Phase’ panels show the phase contrast images of the heterokaryons.
We next made use of interspecies heterokaryon analysis to determine whether xCIRP2 continuously shuttles between the nucleus and cytoplasm (Fig. 5B). In this experiment, the movement of xCIRP2 from human nuclei to mouse nuclei could be monitored based on the accumulation of GFP fusion proteins in the mouse nuclei of heterokaryons. The advantage of using mouse–human heterokaryons lies in the ease with which one can distinguish the two nuclei: the AT-rich mouse centromeres have high affinity for the Hoechst 33342 dye and give rise to bright spots in the murine nucleus (48). Cell fusion was performed in the presence of cycloheximide to inhibit new protein synthesis. Figure 5B shows that xCIRP2 expressed in human nuclei was transported and accumulated to the mouse nuclei. Furthermore, in the similar set of experiments, GFP-RG4 was also accumulated in the mouse nuclei. Based on these results we concluded that xCIRP2 is a novel nucleocytoplasmic shuttling protein and that the RG4 domain has both NLS and NES activities.
Methylation causes cytoplasmic translocation of xCIRP2
Having established that the RG4 domain was a target of xPRMT1 for arginine methylation and was responsible for nucleocytoplasmic shuttling of xCIRP2, we wished to examine the effects of methylation on the intracellular localization of xCIRP2. Accordingly, expression vectors of the GFP-fused xCIRP2 and myc-tagged xPRMT1 were transfected into HeLa S3 cells (Fig. 6). Cytoplasmic levels of xCIRP2 increased upon xPRMT1 expression, while cytoplasmic accumulation was not detected in cells not expressing xPRMT1 (Fig. 6A). xPRMT1 was predominantly detected in the cytoplasm of HeLa cells with the anti-myc antibody. Biochemical fractionation confirmed the increase of xCIRP2 in the cytoplasm (Fig. 6B).
Figure 6.
xCIRP2 accumulates in the cytoplasm of HeLa S3 cells upon arginine methylation by xPRMT1. (A) The expression vector of GFP-xCIRP2-HA alone (left panel) or together with that of myc-tagged xPRMT1 (right panel) was transfected into HeLa cells. Forty-eight hours after transfection, cells were fixed and the myc-xPRMT1 was stained with Alexa Fluor 488-conjugated anti-mouse IgG. The subcellular localization of the GFP-xCIRP2-HA and myc-tagged xPRMT1 was analyzed by fluorescence microscopy. (B) HeLa cells were transfected with the expression vector of GFP-xCIRP2-HA without (lanes 1–3) or with (lanes 4–6) that of xPRMT1. Cytoplasmic fractions (C, lanes 1 and 4), nuclear extracts (NE, lanes 2 and 5) and nuclear pellets (NP, lanes 3 and 6) were prepared and GFP-xCIRP2-HA was detected by immunoblotting with anti-HA antibodies (top panel). The lower graph compares the amount of GFP-xCIRP2-HA in the cytoplasmic fractions and nuclear extracts. The amount of GFP-xCIRP2-HA in nuclear extracts either in the absence or presence of xPRMT1 was set to be 100%. (C) The cytoplasmic fractions were subjected to further fractionation by ultracentrifugation at 100 000 g. GFP-xCIRP2-HA in the supernatant (S100) and the pellet (P100) was detected by immunoblotting as in (B). (D) Cells were incubated in the medium containing 20 µM AdOx for 24 h prior to transfection and AdOx was included in the culture medium until cell fixation. Transfection was performed as in (A).
To examine whether xCIRP2 exists as the component of large particles in HeLa cells, as is the case in Xenopus oocyte (25), the cytoplasmic fraction was subjected to further fractionation by ultracentrifugation at 100 000 g. GFP-xCIRP2 was predominantly found in the pellet (P100) fraction without the xPRMT1 overexpression. This observation suggests that GFP-xCIRP2 in HeLa cells behaved like the endogenous xCIRP2 in the oocytes. When xCIRP2 was accumulated in the cytoplasm upon xPRMT1 expression, xCIRP2 distributed both in the supernatant (S100) and the pellet (P100) fractions (Fig. 6C).
To confirm that the cytoplasmic accumulation of xCIRP2 was a consequence of arginine methylation, we utilized the potent and specific methyl donor inhibitor, AdOx. AdOx increases S-adenosyl-l-homocysteine through its effects on S-adenosyl-l-homocysteine hydrolase, resulting in competitive inhibition of methyltransferases (49–51). HeLa S3 cells were incubated in a medium containing 20 µM AdOx for 24 h prior to transfection and AdOx was continuously included in the culture medium until cell fixation (Fig. 6D). AdOx treatment resulted in the inhibition of the cytoplasmic accumulation of xCIRP2, albeit the distribution pattern of xRPMT1 did not change. It is therefore unlikely that xCIRP2, which continuously shuttles between the nucleus and cytoplasm, would be trapped by xPRMT1 in the cytoplasm. Collectively our results establish that in higher eukaryotes methylation of arginine residues potentially regulates intracellular localization of RNA-binding proteins, which have RGG-type nuclear localization signals, including xCIRP2.
DISCUSSION
In this report, we found that: (i) xCIRP2 continuously shuttles between the nucleus and cytoplasm, (ii) xCIRP2 is methylated by xPRMT1, (iii) the 43-amino acid sequence, RG4, functions as an NSS, moreover, RG4 included the xPRMT1 target(s) for methylation and (iv) methylation by xPRMT1 mediates the cytoplasmic accumulation of xCIRP2. Nucleocytoplasmic shuttling RNA-binding proteins are proposed to associate with (pre-)mRNA in the nucleus, to be involved in RNA export and to affect the subsequent cytoplasmic fate of mRNA (3,13). Based on the high amino acid sequence homology between xCIRP2 and mammalian CIRPs, we speculate that our results can be generalized to mammalian cells in which CIRP may associate with (pre-)mRNA in the nucleus and exit the nucleus accompanying mRNA. Mouse CIRP mediates the cold-induced growth suppression by prolonging the G1 phase (18). It is therefore possible that CIRP binds to a specific set of mRNAs required for normal growth rate at lower temperatures and suppressively affect the fate of those mRNAs.
Although xCIRP2 expressed in HeLa cells accumulated in the nucleus at steady state, treatment of cells with transcriptional inhibitors led to the cytoplasmic accumulation of GFP-xCIRP2 (Fig. 5A). The apparent nuclear accumulation of a continuously shuttling protein can be explained such that the nuclear import rate is much higher than the nuclear export rate of the protein. Indeed, the nuclear export of yeast Npl3p accelerates upon transcriptional inhibition and the authors assume that the duration of transmission of Npl3p through the nucleus is prolonged by association with synthesized RNA (52). Consistent with this, the RG4 sequence that resides in the C-terminal glycine-rich region was responsible for the mRNA-binding activity of xCIRP2 (data not shown). Alternatively, it was proposed that specific post-translational modifications of nuclear transport cargoes or receptors might occur to transduce signals from the mRNA production machinery (53). In any case, it is likely that treatment with transcriptional inhibitors increases the nuclear export rate and/or decreases the import rate of xCIRP2, which consequently allows the detection of cytoplasmic xCIRP2 (also see below).
As mouse CIRP was detected in the nuclei of BALB 3T3 cells, xCIRP2 was found in HeLa nuclei, while it was detected in the cytoplasm of the oocyte (18,25 and this study). It is unlikely that import factors for xCIRP2 are missing in the oocyte. Given that treatment with transcriptional inhibitors induces cytoplasmic accumulation of xCIRP2 in HeLa cells, it is still elusive why xCIRP2 localizes in the cytoplasm of oocytes where transcription by RNA polymerase II is active. If one assumes that xCIRP2 is involved in the metabolism of specific mRNAs, it is intriguing to speculate that active transcription of a specific subset of mRNAs regulates the apparent nuclear localization of xCIRP2. In the oocytes, transcription of these mRNAs might be inactive.
In this study, we showed by yeast two-hybrid analysis that xPRMT1 interacted with xCIRP2. xPRMT1 has significant amino acid sequence homology to other eukaryotic PRMT1s (Fig. 1B). Since the molecular cloning of yeast Rmt1p/Hmt1p, arginine methylation has been the focus of intensive study (54,55). The enzymatic methylation of arginine residues is one of the post-translational modifications exclusively found in eukaryotes (30). Thus, it seems likely that methylation of arginine residues is involved in several fundamental functions, which is a characteristic of eukaryotes. An accurate and complex gene expression in eukaryotes relies on fine control of mRNA metabolism and a variety of RNA-binding proteins containing RGG and/or SR repeats play key roles in this regulation (3,4,13). It is therefore reasonable to expect that predominant substrates of protein arginine methyltransferases are such RNA-binding proteins. In the case of nuclear proteins prepared from rat liver, 65% of NG,NG-dimethylarginine is found in hnRNP proteins (28). Recently, Lee and Bedford identified 10 clones of PRMT1 substrates using high-density protein arrays and, surprisingly, all of them are RNA-binding proteins (56). Furthermore, 4 out of the 10 clones were proven to be CIRP. These results and our experiments presented here provide compelling evidence supporting the assumption that mammalian CIRP and xCIRP2 are primary substrates of PRMT1.
Here, we identified the 43-amino acid sequence, termed RG4, as a methylation site of xCIRP2. Interestingly, the RG4 domain corresponds to a NSS sequence of xCIRP2 and resides in the C-terminal part of xCIRP2 where a major RNA-binding activity of xCIRP2 was located in our preliminary experiment (data not shown). In general, the effects of arginine methylation on RNA-binding activity have not been understood satisfactorily. In previous studies, arginine methylation reduces the activity of hnRNP A1 to bind to coliphage MS2-RNA (57), whereas the RNA-binding activity of a synthetic peptide, corresponding to residues 676–692 of human nucleolin, is not substantially altered upon arginine methylation (58). In addition, methylation by Hmt1p does not affect sequence-specific RNA binding of yeast hnRNP protein, Hrp1p (59). Our preliminary experiments have shown that arginine methylation of the RG4 domain had no effect on the RNA-binding activity of xCIRP2 (data not shown). Further work is definitely necessary to evaluate the effect of methylation on RNA-binding activities.
Our results showing that methylation affects nucleocytoplasmic distribution of RGG-containing RNA-binding proteins have precedents in yeast. At least three hnRNP proteins, Nab2p, Npl3p and Hrp1p, are methylated by Hmt1p/Rmt1p. Although the HMT1 gene is not essential for viability, the nuclear export of these hnRNP proteins is blocked in the absence of HMT1 (55,60). These proteins all contain a glycine- and arginine-rich type nuclear localization signal [(15), also see Fig. 4]. Although considerable studies of arginine methylation are ongoing, it is still poorly understood whether methylation has an impact on intracellular localization of RNA-binding proteins in higher eukaryotes. Our experiments demonstrated that arginine methylation shifted the distribution of xCIRP2 from the nucleus to the cytoplasm. Although endogenous human PRMT1 is active in HeLa cells (38), the translocation of xCIRP2 required xPRMT1 overexpression. As it was shown that protein– protein interactions are regulated by arginine methylation (34,61) it is plausible that the post-translational modification affects the affinities of nuclear import or export receptors to xCIRP2 through the RG4 domain. Our preliminary experiments showed that the GST-RG4 fusion protein was capable of interacting with importin β and transportin, which mediate nuclear import of proteins bearing classical and RG-rich NLSs, respectively (53,62) (data not shown). It is possible that methylation of the RG4 domain alters these interactions.
Mouse CIRP, which might be involved in adaptation to cold stress, is expressed in the nuclei of BALB 3T3 cells following a temperature downshift (18). Human A18 hnRNP, which is equivalent to human CIRP, translocates to the cytoplasm by UV irradiation in RKO cells and plays a protective role against genotoxic stresses (63,64). These observations together with our results raise the possibility that xCIRP2 has separate functions in the nucleus and cytoplasm. The methylation of arginine residues by PRMT1 may be involved in the regulation of such a selective use of CIRP. To test this hypothesis, it must be examined whether PRMT1 activities increase under the conditions such as UV irradiation or presence of transcriptional inhibitors. In our preliminary experiment, however, inhibition of arginine methylation with AdOx did not affect cytoplasmic accumulation of xCIRP2 following treatment with a transcriptional inhibitor (data not shown). Our results suggest that there are at least two mechanisms that lead to cytoplasmic accumulation of xCIRP2: inhibition of transcription and methylation of arginine residues in the RG4 domain.
It is likely that xCIRP2 has multiple functions in various types of cells, being regulated through the methylation of RG4 domain containing RGG repeats. In somatic cells, xCIRP2 might be involved in pre-mRNA processing as has been proposed for hnRNP A1, while in oocytes, xCIRP2 possibly plays a role in translational control through interaction with mRNA and/or ribosomes. In order to clarify the biological significance of xCIRP2, it is necessary to explore the specific conditions that alter the extent of arginine methylation of xCIRP2 in Xenopus oocytes and embryos as well as in somatic cells.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Jun-ichi Miyazaki for providing the pCAGGS vector. This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a grant for a Bioarchitect Research Program from RIKEN.
DDBJ/EMBL/GenBank accession no. AB085173
REFERENCES
- 1.Moore M.J. (2002) Nuclear RNA turnover. Cell, 108, 431–434. [DOI] [PubMed] [Google Scholar]
- 2.Reed R. and Hurt,E. (2002) A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell, 108, 523–531. [DOI] [PubMed] [Google Scholar]
- 3.Shyu A.B. and Wilkinson,M.F. (2000) The double lives of shuttling mRNA binding proteins. Cell, 102, 135–138. [DOI] [PubMed] [Google Scholar]
- 4.Siomi H. and Dreyfuss,G. (1997) RNA-binding proteins as regulators of gene expression. Curr. Opin. Genet. Dev., 7, 345–353. [DOI] [PubMed] [Google Scholar]
- 5.Stutz F. and Rosbash,M. (1998) Nuclear RNA export. Genes Dev., 12, 3303–3319. [DOI] [PubMed] [Google Scholar]
- 6.Dreyfuss G., Matunis,M.J., Pinol-Roma,S. and Burd,C.G. (1993) hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem., 62, 289–321. [DOI] [PubMed] [Google Scholar]
- 7.Daneholt B. (1997) A look at messenger RNP moving through the nuclear pore. Cell, 88, 585–588. [DOI] [PubMed] [Google Scholar]
- 8.Siomi H. and Dreyfuss,G. (1995) A nuclear localization domain in the hnRNP A1 protein. J. Cell Biol., 129, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michael W.M., Choi,M. and Dreyfuss,G. (1995) A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell, 83, 415–422. [DOI] [PubMed] [Google Scholar]
- 10.Weighardt F., Biamonti,G. and Riva,S. (1995) Nucleo-cytoplasmic distribution of human hnRNP proteins: a search for the targeting domains in hnRNP A1. J. Cell Sci., 108 (Pt 2), 545–555. [DOI] [PubMed] [Google Scholar]
- 11.Caceres J.F., Screaton,G.R. and Krainer,A.R. (1998) A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev., 12, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan X.C. and Steitz,J.A. (1998) HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl Acad. Sci. USA, 95, 15293–15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michael W.M. (2000) Nucleocytoplasmic shuttling signals: two for the price of one. Trends Cell Biol., 10, 46–50. [DOI] [PubMed] [Google Scholar]
- 14.Michael W.M., Eder,P.S. and Dreyfuss,G. (1997) The K nuclear shuttling domain: a novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO J., 16, 3587–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun C.Y. and Fu,X.D. (2000) Conserved SR protein kinase functions in nuclear import and its action is counteracted by arginine methylation in Saccharomyces cerevisiae. J. Cell Biol., 150, 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataoka N., Bachorik,J.L. and Dreyfuss,G. (1999) Transportin-SR, a nuclear import receptor for SR proteins. J. Cell Biol., 145, 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard V.W., Michael,W.M., Nakielny,S., Siomi,M.C., Wang,F. and Dreyfuss,G. (1996) A novel receptor-mediated nuclear protein import pathway. Cell, 86, 985–994. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama H., Itoh,K., Kaneko,Y., Kishishita,M., Yoshida,O. and Fujita,J. (1997) A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J. Cell Biol., 137, 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiyama H., Danno,S., Kaneko,Y., Itoh,K., Yokoi,H., Fukumoto,M., Okuno,H., Millan,J.L., Matsuda,T., Yoshida,O. et al. (1998) Decreased expression of cold-inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am. J. Pathol., 152, 289–296. [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatia R., Dube,D.K., Gaur,A., Robertson,D.R., Lemanski,S.L., McLean,M.D. and Lemanski,L.F. (1999) Expression of axolotl RNA-binding protein during development of the Mexican axolotl. Cell Tissue Res., 297, 283–290. [DOI] [PubMed] [Google Scholar]
- 21.Nishiyama H., Higashitsuji,H., Yokoi,H., Itoh,K., Danno,S., Matsuda,T. and Fujita,J. (1997) Cloning and characterization of human CIRP (cold-inducible RNA-binding protein) cDNA and chromosomal assignment of the gene. Gene, 204, 115–120. [DOI] [PubMed] [Google Scholar]
- 22.Saito T., Sugimoto,K., Adachi,Y., Wu,Q. and Mori,K.J. (2000) Cloning and characterization of amphibian cold inducible RNA-binding protein. Comp. Biochem. Physiol. B Biochem. Mol. Biol., 125, 237–245. [DOI] [PubMed] [Google Scholar]
- 23.Uochi T. and Asashima,M. (1998) XCIRP (Xenopus homolog of cold-inducible RNA-binding protein) is expressed transiently in developing pronephros and neural tissue. Gene, 211, 245–250. [DOI] [PubMed] [Google Scholar]
- 24.Xue J.H., Nonoguchi,K., Fukumoto,M., Sato,T., Nishiyama,H., Higashitsuji,H., Itoh,K. and Fujita,J. (1999) Effects of ischemia and H2O2 on the cold stress protein CIRP expression in rat neuronal cells. Free Radic. Biol. Med., 27, 1238–1244. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto K., Aoki,K., Dohmae,N., Takio,K. and Tsujimoto,M. (2000) CIRP2, a major cytoplasmic RNA-binding protein in Xenopus oocytes. Nucleic Acids Res., 28, 4689–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Y., Kok,K.H., Xu,R.H., Kwok,K.H., Tay,D., Fung,P.C., Kung,H.F. and Lin,M.C. (2000) Maternal cold inducible RNA binding protein is required for embryonic kidney formation in Xenopus laevis. FEBS Lett., 482, 37–43. [DOI] [PubMed] [Google Scholar]
- 27.McBride A.E. and Silver,P.A. (2001) State of the arg: protein methylation at arginine comes of age. Cell, 106, 5–8. [DOI] [PubMed] [Google Scholar]
- 28.Boffa L.C., Karn,J., Vidali,G. and Allfrey,V.G. (1977) Distribution of NG, NG,-dimethylarginine in nuclear protein fractions. Biochem. Biophys. Res. Commun., 74, 969–976. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A., Williams,K.R. and Szer,W. (1986) Purification and domain structure of core hnRNP proteins A1 and A2 and their relationship to single-stranded DNA-binding proteins. J. Biol. Chem., 261, 11266–11273. [PubMed] [Google Scholar]
- 30.Liu Q. and Dreyfuss,G. (1995) In vivo and in vitro arginine methylation of RNA-binding proteins. Mol. Cell. Biol., 15, 2800–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Huang,Z.Q., Xia,L., Feng,Q., Erdjument-Bromage,H., Strahl,B.D., Briggs,S.D., Allis,C.D., Wong,J., Tempst,P. et al. (2001) Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science, 293, 853–857. [DOI] [PubMed] [Google Scholar]
- 32.Tang J., Frankel,A., Cook,R.J., Kim,S., Paik,W.K., Williams,K.R., Clarke,S. and Herschman,H.R. (2000) PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J. Biol. Chem., 275, 7723–7730. [DOI] [PubMed] [Google Scholar]
- 33.Smith J.J., Rucknagel,K.P., Schierhorn,A., Tang,J., Nemeth,A., Linder,M., Herschman,H.R. and Wahle,E. (1999) Unusual sites of arginine methylation in Poly(A)-binding protein II and in vitro methylation by protein arginine methyltransferases PRMT1 and PRMT3. J. Biol. Chem., 274, 13229–13234. [DOI] [PubMed] [Google Scholar]
- 34.Mowen K.A., Tang,J., Zhu,W., Schurter,B.T., Shuai,K., Herschman,H.R. and David,M. (2001) Arginine methylation of STAT1 modulates IFNalpha/beta-induced transcription. Cell, 104, 731–741. [DOI] [PubMed] [Google Scholar]
- 35.Abramovich C., Yakobson,B., Chebath,J. and Revel,M. (1997) A protein-arginine methyltransferase binds to the intracytoplasmic domain of the IFNAR1 chain in the type I interferon receptor. EMBO J., 16, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S., Merrill,B.M., Rajpurohit,R., Kumar,A., Stone,K.L., Papov,V.V., Schneiders,J.M., Szer,W., Wilson,S.H., Paik,W.K. et al. (1997) Identification of N(G)-methylarginine residues in human heterogeneous RNP protein A1: Phe/Gly-Gly-Gly-Arg-Gly-Gly-Gly/Phe is a preferred recognition motif. Biochemistry, 36, 5185–5192. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto K., Nagata,K., Miyaji-Yamaguchi,M., Kikuchi,A. and Tsujimoto,M. (1999) Sperm chromatin decondensation by template activating factor I through direct interaction with basic proteins. Mol. Cell. Biol., 19, 6940–6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang J., Gary,J.D., Clarke,S. and Herschman,H.R. (1998) PRMT 3, a type I protein arginine N-methyltransferase that differs from PRMT1 in its oligomerization, subcellular localization, substrate specificity and regulation. J. Biol. Chem., 273, 16935–16945. [DOI] [PubMed] [Google Scholar]
- 39.Niwa H., Yamamura,K. and Miyazaki,J. (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene, 108, 193–199. [DOI] [PubMed] [Google Scholar]
- 40.Scott H.S., Antonarakis,S.E., Lalioti,M.D., Rossier,C., Silver,P.A. and Henry,M.F. (1998) Identification and characterization of two putative human arginine methyltransferases (HRMT1L1 and HRMT1L2). Genomics, 48, 330–340. [DOI] [PubMed] [Google Scholar]
- 41.Henry M.F. and Silver,P.A. (1996) A novel methyltransferase (Hmt1p) modifies poly(A)+-RNA-binding proteins. Mol. Cell. Biol., 16, 3668–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajpurohit R., Lee,S.O., Park,J.O., Paik,W.K. and Kim,S. (1994) Enzymatic methylation of recombinant heterogeneous nuclear RNP protein A1. Dual substrate specificity for S-adenosylmethionine:histone-arginine N-methyltransferase. J. Biol. Chem., 269, 1075–1082. [PubMed] [Google Scholar]
- 43.Truant R., Fridell,R.A., Benson,R.E., Bogerd,H. and Cullen,B.R. (1998) Identification and functional characterization of a novel nuclear localization signal present in the yeast Nab2 poly(A)+ RNA binding protein. Mol. Cell. Biol., 18, 1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gama-Carvalho M., Carvalho,M.P., Kehlenbach,A., Valcarcel,J. and Carmo-Fonseca,M. (2001) Nucleocytoplasmic shuttling of heterodimeric splicing factor U2AF. J. Biol. Chem., 276, 13104–13112. [DOI] [PubMed] [Google Scholar]
- 45.Bouvet P., Diaz,J.J., Kindbeiter,K., Madjar,J.J. and Amalric,F. (1998) Nucleolin interacts with several ribosomal proteins through its RGG domain. J. Biol. Chem., 273, 19025–19029. [DOI] [PubMed] [Google Scholar]
- 46.Pinol-Roma S. and Dreyfuss,G. (1992) Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature, 355, 730–732. [DOI] [PubMed] [Google Scholar]
- 47.Egyhazi E., Pigon,A. and Rydlander,L. (1982) 5,6-Dichlororibofuranosylbenzimidazole inhibits the rate of transcription initiation in intact Chironomus cells. Eur. J. Biochem., 122, 445–451. [DOI] [PubMed] [Google Scholar]
- 48.Moser F.G., Dorman,B.P. and Ruddle,F.H. (1975) Mouse-human heterokaryon analysis with a 33258 Hoechst-Giemsa technique. J. Cell Biol., 66, 676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liteplo R.G. (1988) DNA (cytosine) methylation in murine and human tumor cell lines treated with S-adenosylhomocysteine hydrolase inhibitors. Cancer Lett., 39, 319–327. [DOI] [PubMed] [Google Scholar]
- 50.Bartel R.L. and Borchardt,R.T. (1984) Effects of adenosine dialdehyde on S-adenosylhomocysteine hydrolase and S-adenosylmethionine-dependent transmethylations in mouse L929 cells. Mol. Pharmacol., 25, 418–424. [PubMed] [Google Scholar]
- 51.Hoffman J.L. (1979) Transmethylation. Elsevier Science Publishing Co., New York.
- 52.Liu Y., Guo,W., Tartakoff,P.Y. and Tartakoff,A.M. (1999) A Crm1p-independent nuclear export path for the mRNA-associated protein, Npl3p/Mtr13p. Proc. Natl Acad. Sci. USA, 96, 6739–6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakielny S. and Dreyfuss,G. (1999) Transport of proteins and RNAs in and out of the nucleus. Cell, 99, 677–690. [DOI] [PubMed] [Google Scholar]
- 54.Gary J.D., Lin,W.J., Yang,M.C., Herschman,H.R. and Clarke,S. (1996) The predominant protein-arginine methyltransferase from Saccharomyces cerevisiae. J. Biol. Chem., 271, 12585–12594. [DOI] [PubMed] [Google Scholar]
- 55.Green D.M., Marfatia,K.A., Crafton,E.B., Zhang,X., Cheng,X. and Corbett,A.H. (2002) Nab2p is required for poly(A) RNA export in Saccharomyces cerevisiae and is regulated by arginine methylation via Hmt1p. J. Biol. Chem., 277, 7752–7760. [DOI] [PubMed] [Google Scholar]
- 56.Lee J. and Bedford,M.T. (2002) PABP1 identified as an arginine methyltransferase substrate using high-density protein arrays. EMBO Rep., 3, 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajpurohit R., Paik,W.K. and Kim,S. (1994) Effect of enzymic methylation of heterogeneous ribonucleoprotein particle A1 on its nucleic-acid binding and controlled proteolysis. Biochem. J., 304 (Pt 3), 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raman B., Guarnaccia,C., Nadassy,K., Zakhariev,S., Pintar,A., Zanuttin,F., Frigyes,D., Acatrinei,C., Vindigni,A., Pongor,G. et al. (2001) N(omega)-arginine dimethylation modulates the interaction between a Gly/Arg-rich peptide from human nucleolin and nucleic acids. Nucleic Acids Res., 29, 3377–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valentini S.R., Weiss,V.H. and Silver,P.A. (1999) Arginine methylation and binding of Hrp1p to the efficiency element for mRNA 3′-end formation. RNA, 5, 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen E.C., Henry,M.F., Weiss,V.H., Valentini,S.R., Silver,P.A. and Lee,M.S. (1998) Arginine methylation facilitates the nuclear export of hnRNP proteins. Genes Dev., 12, 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friesen W.J., Massenet,S., Paushkin,S., Wyce,A. and Dreyfuss,G. (2001) SMN, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Mol. Cell., 7, 1111–1117. [DOI] [PubMed] [Google Scholar]
- 62.Gorlich D. and Kutay,U. (1999) Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell. Dev. Biol., 15, 607–660. [DOI] [PubMed] [Google Scholar]
- 63.Sheikh M.S., Carrier,F., Papathanasiou,M.A., Hollander,M.C., Zhan,Q., Yu,K. and Fornace,A.J.,Jr (1997) Identification of several human homologs of hamster DNA damage-inducible transcripts. Cloning and characterization of a novel UV-inducible cDNA that codes for a putative RNA-binding protein. J. Biol. Chem., 272, 26720–26726. [DOI] [PubMed] [Google Scholar]
- 64.Yang C. and Carrier,F. (2001) The UV-inducible RNA-binding protein A18 (A18 hnRNP) plays a protective role in the genotoxic stress response. J. Biol. Chem., 276, 47277–47284. [DOI] [PubMed] [Google Scholar]
- 65.Feldmann H., Aigle,M., Aljinovic,G., Andre,B., Baclet,M.C., Barthe,C., Baur,A., Becam,A.M., Biteau,N., Boles,E. et al. (1994) Complete DNA sequence of yeast chromosome II. EMBO J., 13, 5795–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goffeau A., Barrell,B.G., Bussey,H., Davis,R.W., Dujon,B., Feldmann,H., Galibert,F., Hoheisel,J.D., Jacq,C., Johnston,M. et al. (1996) Life with 6000 genes. Science, 274, 546, 563–547. [DOI] [PubMed] [Google Scholar]
- 67.Burd C.G. and Dreyfuss,G. (1994) Conserved structures and diversity of functions of RNA-binding proteins. Science, 265, 615–621. [DOI] [PubMed] [Google Scholar]