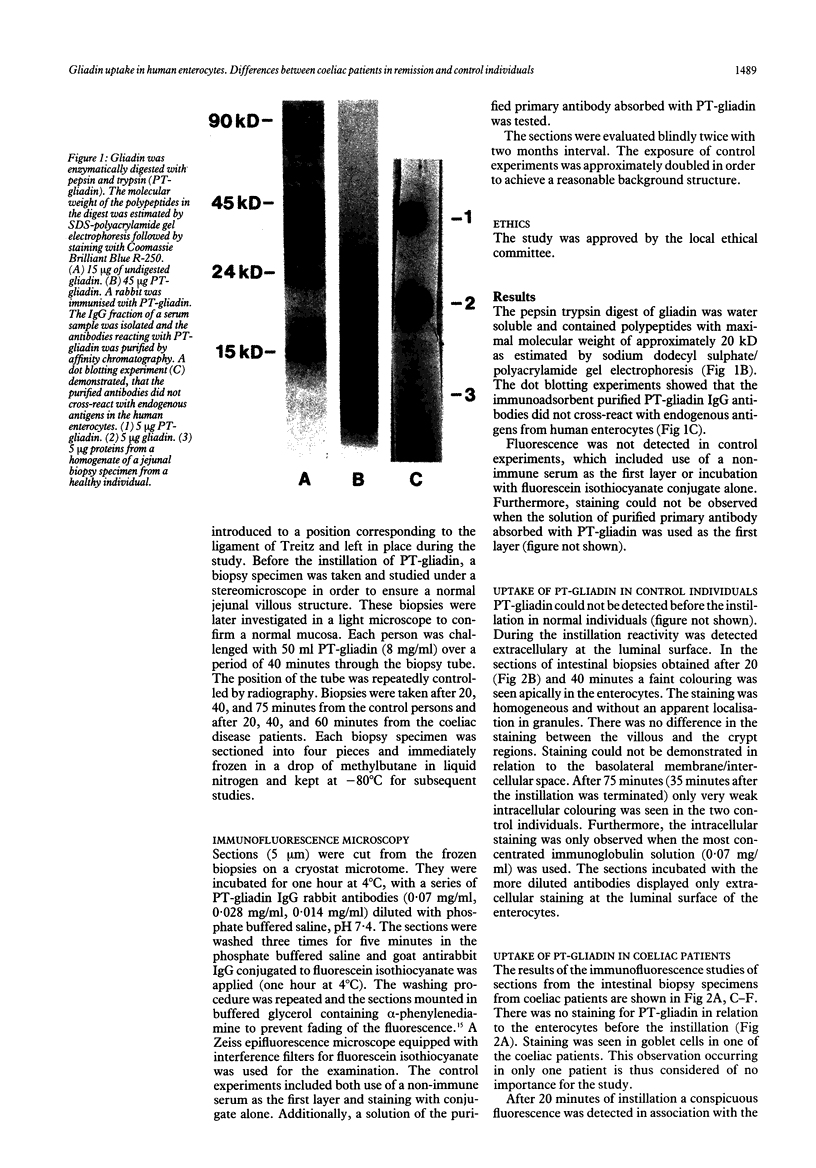
Abstract
The pepsin trypsin digest of the wheat prolamin gliadin (PT-gliadin) is deleterious to the small intestinal mucosa of coeliac patients. The handling of PT-gliadin by the intestinal epithelium in coeliac patients in remission and control individuals was investigated by in vivo instillation of PT-gliadin. The uptake of PT-gliadin was monitored by immunofluorescence microscopy of intestinal biopsy specimens, using affinity purified PT-gliadin antibodies. Control individuals show weak staining in the apical region of the enterocytes thereby showing an uptake of PT-gliadin. Coeliac patients have a conspicuous fluorescence in relation to the lateral membrane/intercellular space of enterocytes and intense staining intracellularly in the apical region. There is only weak staining in the enterocytes after the instillation was terminated, indicating an intracellular clearance. The study shows that normal enterocytes are able to take up PT-gliadin. The increased uptake in coeliac patients might be of importance for the pathogenesis either by direct toxicity or by presentation to immunocompetent cells. Furthermore, the results are in agreement with the suggestion of a functional alteration in the zonula occludens in the intestinal epithelium of coeliac patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand B. S., Piris J., Jerrome D. W., Offord R. E., Truelove S. C. The timing of histological damage following a single challenge with gluten in treated coeliac disease. Q J Med. 1981;50(197):83–94. [PubMed] [Google Scholar]
- Bjarnason I., Peters T. J., Veall N. A persistent defect in intestinal permeability in coeliac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet. 1983 Feb 12;1(8320):323–325. doi: 10.1016/s0140-6736(83)91628-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bramble M. G., Zucoloto S., Wright N. A., Record C. O. Acute gluten challenge in treated adult coeliac disease: a morphometric and enzymatic study. Gut. 1985 Feb;26(2):169–174. doi: 10.1136/gut.26.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein H. D., Haeffner L. J., Kowlessar O. D. Enzymatic digestion of gliadin: the effect of the resultant peptides in adult celiac disease. Clin Chim Acta. 1966 Aug;14(2):141–155. doi: 10.1016/0009-8981(66)90080-5. [DOI] [PubMed] [Google Scholar]
- Bruce G., Woodley J. F., Swan C. H. Breakdown of gliadin peptides by intestinal brush borders from coeliac patients. Gut. 1984 Sep;25(9):919–924. doi: 10.1136/gut.25.9.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude P., Goodenough D. A. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J Cell Biol. 1973 Aug;58(2):390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell H. J., Rolles C. J. Further evidence of a primary mucosal defect in coeliac disease. Gut. 1978 Apr;19(4):253–259. doi: 10.1136/gut.19.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. G., Bridges M. A. Coeliac disease: a critical review of aetiology and pathogenesis. Clin Chim Acta. 1987 Feb 27;163(1):1–40. doi: 10.1016/0009-8981(87)90031-3. [DOI] [PubMed] [Google Scholar]
- Douglas A. P., Booth C. C. Digestion of gluten peptides by normal human jejunal mucosa and by mucosa from patients with adult coeliac disease. Clin Sci. 1970 Jan;38(1):11–25. doi: 10.1042/cs0380011. [DOI] [PubMed] [Google Scholar]
- FRAZER A. C., FLETCHER R. F., ROSS C. A., SHAW B., SAMMONS H. G., SCHNEIDER R. Gluten-induced enteropathy: the effect of partially digested gluten. Lancet. 1959 Sep 5;2(7097):252–255. doi: 10.1016/s0140-6736(59)92051-3. [DOI] [PubMed] [Google Scholar]
- Friis S. U. Enzyme-linked immunosorbent assay for quantitation of cereal proteins toxic in coeliac disease. Clin Chim Acta. 1988 Dec 30;178(3):261–270. doi: 10.1016/0009-8981(88)90234-3. [DOI] [PubMed] [Google Scholar]
- Friis S. U., Norén O., Sjöström H., Gudmand-Høyer E. Patients with coeliac disease have a characteristic gliadin antibody pattern. Clin Chim Acta. 1986 Mar 16;155(2):133–141. doi: 10.1016/0009-8981(86)90274-3. [DOI] [PubMed] [Google Scholar]
- Friis S. U., Sjöström H., Norén O., Rüdiger N., Anthonsen D. The prolamin antibody reactivity against hordein polypeptides in sera from patients with coeliac disease. Clin Chim Acta. 1988 Sep 15;176(3):241–250. doi: 10.1016/0009-8981(88)90183-0. [DOI] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Leigh R. J., Marsh M. N., Crowe P., Kelly C., Garner V., Gordon D. Studies of intestinal lymphoid tissue. IX. Dose-dependent, gluten-induced lymphoid infiltration of coeliac jejunal epithelium. Scand J Gastroenterol. 1985 Aug;20(6):715–719. doi: 10.3109/00365528509089201. [DOI] [PubMed] [Google Scholar]
- Pittman F. E., Pollitt R. J. Studies of jejunal mucosal digestion of peptic-tryptic digests of wheat protein in coeliac disease. Gut. 1966 Aug;7(4):368–371. doi: 10.1136/gut.7.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin W., Ross L. L., Sleisenger M. H., Weser E. An electron microscopic study of adult celiac disease. Lab Invest. 1966 Nov;15(11):1720–1747. [PubMed] [Google Scholar]