Abstract
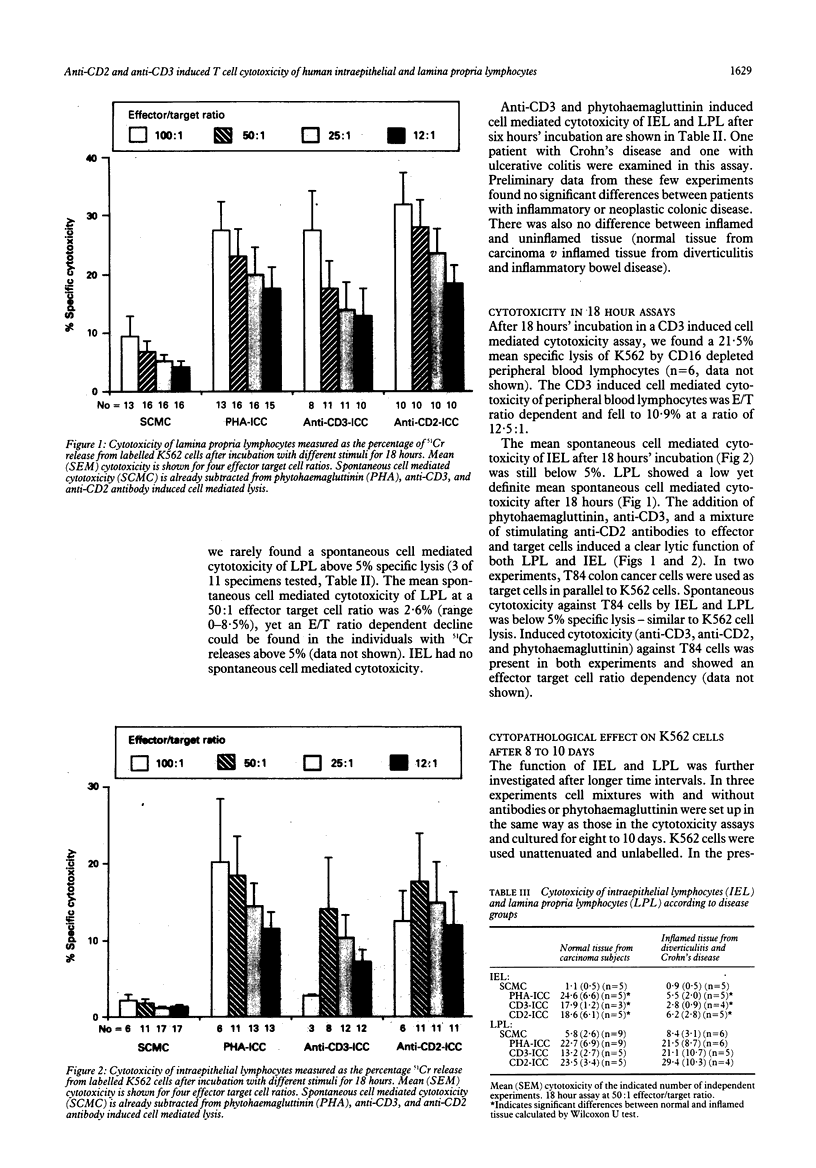
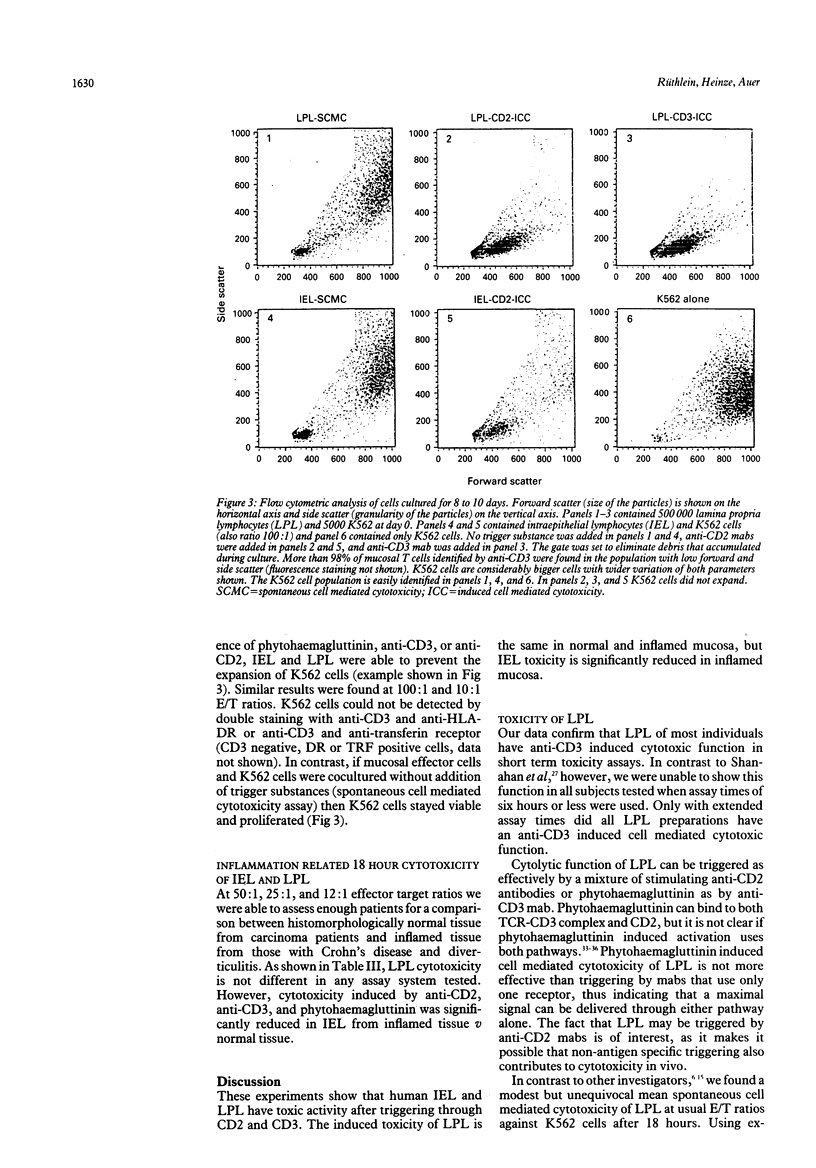
The effector function of immunocompetent cells in the gut mucosa has not yet been defined. The cytotoxic function of these cells might be important in the normal immune response and could be relevant to the mucosal damage seen in inflammatory conditions. The cytotoxic function of isolated intraepithelial and lamina propria mononuclear cells in six and 18 hour assays after the addition of various stimuli that interact with the human leukocyte antigens CD2 and CD3 on the mucosal effector cells was investigated. T cell phenotypes were determined using CD4, CD8, and HML1 to characterise cells of the appropriate compartments. Anti-CD3 and phytohaemagglutinin can induce toxic activity of lamina propria lymphocytes in most individuals after six hours and in all individuals after 18 hours. Anti-CD2, anti-CD3, and phytohaemagglutinin are similarly effective at triggering lamina propria lymphocytes. Intraepithelial lymphocytes contain predominantly CD8 and HML1 positive T cells, differentiating phenotypically intraepithelial lymphocytes from lamina propria lymphocytes. Intraepithelial lymphocytes are not cytotoxic at six hours, but have a toxic function comparable with lamina propria lymphocytes after 18 hours with all three triggers. Intraepithelial lymphocytes from inflamed mucosa (Crohn's disease and diverticulitis) mediate significantly reduced cytotoxicity in vitro compared with normal mucosa, whereas lamina propria lymphocyte toxicity is not different. Reduced numbers of cytotoxic cells and reduced reactivity to the trigger substances used after in vivo activation or cold target inhibition could explain the observed differences between intraepithelial lymphocytes from inflamed and uninflamed mucosa. Changes in cell mediated cytotoxicity of intraepithelial lymphocytes and lamina propria lymphocytes may be involved in the mucosal damage in these inflammatory conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud-Battandier F., Bundy B. M., O'Neill M., Bienenstock J., Nelson D. L. Cytotoxic activities of gut mucosal lymphoid cells in guinea pigs. J Immunol. 1978 Sep;121(3):1059–1065. [PubMed] [Google Scholar]
- Auer I. O., Rüthlein J., Röder A., Reineke C., Grudzinsky K. Untersuchungen der intestinalen spontanen zellvermittelten Zytotoxizität und ihrer Immunregulation bei Patienten mit chronisch-entzündlichen Darmerkrankungen und Kontrollen. Immun Infekt. 1986 Feb;14(1):22–23. [PubMed] [Google Scholar]
- Bookman M. A., Bull D. M. Characteristics of isolated intestinal mucosal lymphoid cells in inflammatory bowel disease. Gastroenterology. 1979 Sep;77(3):503–510. [PubMed] [Google Scholar]
- Brandtzaeg P., Halstensen T. S., Kett K., Krajci P., Kvale D., Rognum T. O., Scott H., Sollid L. M. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989 Dec;97(6):1562–1584. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985 Jan;26(1):81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Jarry A., Brousse N., Lisowska-Grospierre B., Guy-Grand D., Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987 Sep;17(9):1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- Chiba M., Bartnik W., ReMine S. G., Thayer W. R., Shorter R. G. Human colonic intraepithelial and lamina proprial lymphocytes: cytotoxicity in vitro and the potential effects of the isolation method on their functional properties. Gut. 1981 Mar;22(3):177–186. doi: 10.1136/gut.22.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Pizzo P., Zanovello P., Bronte V., Collavo D. Extracellular ATP as a possible mediator of cell-mediated cytotoxicity. Immunol Today. 1990 Aug;11(8):274–277. doi: 10.1016/0167-5699(90)90111-l. [DOI] [PubMed] [Google Scholar]
- Ebert E. C. Proliferative responses of human intraepithelial lymphocytes to various T-cell stimuli. Gastroenterology. 1989 Dec;97(6):1372–1381. doi: 10.1016/0016-5085(89)90379-x. [DOI] [PubMed] [Google Scholar]
- Ebert E. C., Roberts A. I., Brolin R. E., Raska K. Examination of the low proliferative capacity of human jejunal intraepithelial lymphocytes. Clin Exp Immunol. 1986 Jul;65(1):148–157. [PMC free article] [PubMed] [Google Scholar]
- Falchuk Z. M., Barnhard E., Machado I. Human colonic mononuclear cells: studies of cytotoxic function. Gut. 1981 Apr;22(4):290–294. doi: 10.1136/gut.22.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C., Tubbs R. R., Youngman K. R. Human intestinal mucosal mononuclear cells exhibit lymphokine-activated killer cell activity. Gastroenterology. 1985 Mar;88(3):625–637. doi: 10.1016/0016-5085(85)90130-1. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Dow E. L., Selby W. S., Strickland R. G., Jewell D. P. Natural killer cells and spontaneous cell-mediated cytotoxicity in the human intestine. Clin Exp Immunol. 1984 May;56(2):438–444. [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Dow E. L., Selby W. S., Strickland R. G., Jewell D. P. Natural killer cells and spontaneous cell-mediated cytotoxicity in the human intestine. Clin Exp Immunol. 1984 May;56(2):438–444. [PMC free article] [PubMed] [Google Scholar]
- Hogan P. G., Hapel A. J., Doe W. F. Lymphokine-activated and natural killer cell activity in human intestinal mucosa. J Immunol. 1985 Sep;135(3):1731–1738. [PubMed] [Google Scholar]
- Kanellopoulos J. M., De Petris S., Leca G., Crumpton M. J. The mitogenic lectin from Phaseolus vulgaris does not recognize the T3 antigen of human T lymphocytes. Eur J Immunol. 1985 May;15(5):479–486. doi: 10.1002/eji.1830150512. [DOI] [PubMed] [Google Scholar]
- Leca G., Boumsell L., Fabbi M., Reinherz E. L., Kanellopoulos J. M. The sheep erythrocyte receptor and both alpha and beta chains of the human T-lymphocyte antigen receptor bind the mitogenic lectin (phytohaemagglutinin) from Phaseolus vulgaris. Scand J Immunol. 1986 May;23(5):535–544. doi: 10.1111/j.1365-3083.1986.tb01985.x. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Bragdon M. J., Kodner I. J., Bertovich M. J. Deficient cell-mediated cytotoxicity and hyporesponsiveness to interferon and mitogenic lectin activation by inflammatory bowel disease peripheral blood and intestinal mononuclear cells. Gastroenterology. 1986 Jan;90(1):6–11. doi: 10.1016/0016-5085(86)90067-3. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Franklin G. O., Jenkins K. M., Kodner I. J., Nash G. S., Weinrieb I. J. Human intestinal mononuclear cells. I. Investigation of antibody-dependent, lectin-induced, and spontaneous cell-mediated cytotoxic capabilities. Gastroenterology. 1980 Jan;78(1):47–56. [PubMed] [Google Scholar]
- Mentzer S. J., Barbosa J. A., Burakoff S. J. T3 monoclonal antibody activation of nonspecific cytolysis: a mechanism of CTL inhibition. J Immunol. 1985 Jul;135(1):34–38. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Mowat A. M. Human intraepithelial lymphocytes. Springer Semin Immunopathol. 1990;12(2-3):165–190. doi: 10.1007/BF00197504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flynn K., Knott L. J., Russul-Saib M., Abdul-Gaffar R., Morgan G., Beverley P. C., Linch D. C. CD2 and CD3 antigens mobilize Ca2+ independently. Eur J Immunol. 1986 May;16(5):580–584. doi: 10.1002/eji.1830160521. [DOI] [PubMed] [Google Scholar]
- O'Flynn K., Krensky A. M., Beverley P. C., Burakoff S. J., Linch D. C. Phytohaemagglutinin activation of T cells through the sheep red blood cell receptor. Nature. 1985 Feb 21;313(6004):686–687. doi: 10.1038/313686a0. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Lectin-dependent and anti-CD3 induced cytotoxicity are preferentially mediated by peripheral blood cytotoxic T lymphocytes expressing Leu-7 antigen. J Immunol. 1986 Mar 1;136(5):1579–1585. [PubMed] [Google Scholar]
- Pirzer U. C., Schürmann G., Post S., Betzler M., Meuer S. C. Differential responsiveness to CD3-Ti vs. CD2-dependent activation of human intestinal T lymphocytes. Eur J Immunol. 1990 Oct;20(10):2339–2342. doi: 10.1002/eji.1830201025. [DOI] [PubMed] [Google Scholar]
- Qiao L., Schürmann G., Betzler M., Meuer S. C. Activation and signaling status of human lamina propria T lymphocytes. Gastroenterology. 1991 Dec;101(6):1529–1536. doi: 10.1016/0016-5085(91)90388-2. [DOI] [PubMed] [Google Scholar]
- Roche J. K., Fiocchi C., Youngman K. Sensitization to epithelial antigens in chronic mucosal inflammatory disease. Characterization of human intestinal mucosa-derived mononuclear cells reactive with purified epithelial cell-associated components in vitro. J Clin Invest. 1985 Feb;75(2):522–530. doi: 10.1172/JCI111728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüthlein J., Heinze G. Anti-CD3- or PHA-induced cytotoxicity in human intraepithelial and lamina propria lymphocytes. Immunol Res. 1991;10(3-4):226–229. doi: 10.1007/BF02919697. [DOI] [PubMed] [Google Scholar]
- Rüthlein J., James S. P., Strober W. Role of CD2 in activation and cytotoxic function of CD8/Leu-7-positive T cells. J Immunol. 1988 Dec 1;141(11):3791–3797. [PubMed] [Google Scholar]
- Schrezenmeier H., Kurrle R., Wagner H., Fleischer B. Activation of human T lymphocytes. III. Triggering of bystander cytotoxicity in cytotoxic T cell clones by antibodies against the T3 antigen or by a calcium ionophore. Eur J Immunol. 1985 Oct;15(10):1019–1024. doi: 10.1002/eji.1830151011. [DOI] [PubMed] [Google Scholar]
- Shanahan F., Brogan M., Targan S. Human mucosal cytotoxic effector cells. Gastroenterology. 1987 Jun;92(6):1951–1957. doi: 10.1016/0016-5085(87)90629-9. [DOI] [PubMed] [Google Scholar]
- Shanahan F., Deem R., Nayersina R., Leman B., Targan S. Human mucosal T-cell cytotoxicity. Gastroenterology. 1988 Apr;94(4):960–967. doi: 10.1016/0016-5085(88)90554-9. [DOI] [PubMed] [Google Scholar]
- Shanahan F., Leman B., Deem R., Niederlehner A., Brogan M., Targan S. Enhanced peripheral blood T-cell cytotoxicity in inflammatory bowel disease. J Clin Immunol. 1989 Jan;9(1):55–64. doi: 10.1007/BF00917128. [DOI] [PubMed] [Google Scholar]
- Shorter R. G., McGill D. B., Bahn R. C. Cytotoxicity of mononuclear cells for autologous colonic epithelial cells in colonic diseases. Gastroenterology. 1984 Jan;86(1):13–22. [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Befus A. D., Clark D. A., Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982 Jun 1;155(6):1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan S. R., Kagnoff M. F., Brogan M. D., Shanahan F. Immunologic mechanisms in intestinal diseases. Ann Intern Med. 1987 Jun;106(6):853–870. doi: 10.7326/0003-4819-106-6-853. [DOI] [PubMed] [Google Scholar]
- Taunk J., Roberts A. I., Ebert E. C. Spontaneous cytotoxicity of human intraepithelial lymphocytes against epithelial cell tumors. Gastroenterology. 1992 Jan;102(1):69–75. doi: 10.1016/0016-5085(92)91785-3. [DOI] [PubMed] [Google Scholar]
- Young J. D. Killing of target cells by lymphocytes: a mechanistic view. Physiol Rev. 1989 Jan;69(1):250–314. doi: 10.1152/physrev.1989.69.1.250. [DOI] [PubMed] [Google Scholar]
- van Seventer G. A., Kuijpers K. C., van Lier R. A., de Groot E. R., Aarden L. A., Melief C. J. Mechanism of inhibition and induction of cytolytic activity in cytotoxic T lymphocytes by CD3 monoclonal antibodies. J Immunol. 1987 Oct 15;139(8):2545–2550. [PubMed] [Google Scholar]


