Abstract
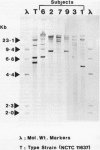
Nine members of a family with a high incidence of duodenal ulcer disease were studied by interview, examination of hospital records, endoscopy, and antral biopsy. Helicobacter pylori was confirmed by CLO test, histology and culture. DNA extraction from pure isolates of H pylori was possible in six family members and strain typing was performed by restriction fragment length polymorphism. DNA restriction digestion was followed by vacublotting and then DNA hybridisation, using a cDNA probe complimentary to H pylori rRNA cistrons. Eight of the nine family members were H pylori positive by CLO test and histology. Five had duodenal ulcer disease. Three family members (one from each generation) harboured clonal variants of a single parent strain of H pylori but only two had duodenal disease. The other three members harboured different strains. Intrafamilial clustering of clonal variants of H pylori occurs in some duodenal ulcer disease families. Family members however, may develop duodenal disease irrespective of the colonising strain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cave D. R., Vargas M. Effect of a Campylobacter pylori protein on acid secretion by parietal cells. Lancet. 1989 Jul 22;2(8656):187–189. doi: 10.1016/s0140-6736(89)90372-3. [DOI] [PubMed] [Google Scholar]
- Cleaning and disinfection of equipment for gastrointestinal flexible endoscopy: interim recommendations of a Working Party of the British Society of Gastroenterology. Gut. 1988 Aug;29(8):1134–1151. doi: 10.1136/gut.29.8.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas M., Owen R. J., Bickley J., Morgan D. R. Molecular techniques for studying the epidemiology of infection by Helicobacter pylori. Scand J Gastroenterol Suppl. 1991;181:20–32. doi: 10.3109/00365529109093204. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Drumm B., Perez-Perez G. I., Blaser M. J., Sherman P. M. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990 Feb 8;322(6):359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman D., Newell D. G., Fullerton F., Yarnell J. W., Stacey A. R., Wald N., Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991 Jun 1;302(6788):1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin C. S. Duodenal ulcer, Campylobacter pylori, and the "leaking roof" concept. Lancet. 1988 Dec 24;2(8626-8627):1467–1469. doi: 10.1016/s0140-6736(88)90942-7. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Malaty H. M., Evans D. G., Evans D. J., Jr, Klein P. D., Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991 Jun;100(6):1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- Levi S., Beardshall K., Haddad G., Playford R., Ghosh P., Calam J. Campylobacter pylori and duodenal ulcers: the gastrin link. Lancet. 1989 May 27;1(8648):1167–1168. doi: 10.1016/s0140-6736(89)92752-9. [DOI] [PubMed] [Google Scholar]
- Morgan D. R., Mathewson J. J., Freedman R., Kraft W. G. Evaluation of a selective enrichment technique for the isolation of Campylobacter pylori. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):303–306. doi: 10.1016/0378-1097(90)90302-7. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Bickley J., Costas M., Morgan D. R. Genomic variation in Helicobacter pylori: application to identification of strains. Scand J Gastroenterol Suppl. 1991;181:43–50. doi: 10.3109/00365529109093207. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Fraser J., Costas M., Morgan D., Morgan D. R. Signature patterns of DNA restriction fragments of Helicobacter pylori before and after treatment. J Clin Pathol. 1990 Aug;43(8):646–649. doi: 10.1136/jcp.43.8.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T., Pounder R. E., Nwokolo C. U., Lanzon-Miller S., Evans D. G., Graham D. Y., Evans D. J., Jr Inappropriate hypergastrinaemia in asymptomatic healthy subjects infected with Helicobacter pylori. Gut. 1990 May;31(5):522–525. doi: 10.1136/gut.31.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Mine K., Nakai Y., Mishima N., Nakagawa T. Serum pepsinogen I concentrations in peptic ulcer patients in relation to ulcer location and stage. Gut. 1991 Aug;32(8):849–852. doi: 10.1136/gut.32.8.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P., Gottrand F., Pernes P., Husson M. A., Beju A., Leclerc H., Farriaux J. P. Helicobacter pylori infection in cohabiting children. Lancet. 1991 Apr 6;337(8745):848–848. doi: 10.1016/0140-6736(91)92550-l. [DOI] [PubMed] [Google Scholar]