Abstract
1 This study presents the results of the preliminary screening of vigabatrin as add-on therapy in an open, non-controlled multicentre study in children with refractory epilepsy.
2 There were 135 children, with an age range of 2 months-12 years. Main seizure type was partial in 42%, generalized in 29%, Lennox-Gastaut syndrome in 19% and West syndrome in 10%.
3 Vigabatrin was added onto current antiepileptic treatment in an initially recommended dose of 40-80 mg kg-1 day-1. However, the doses were frequently increased when tolerance allowed it, and the final mean dose used was 87 mg kg-1 day-1 (27-600).
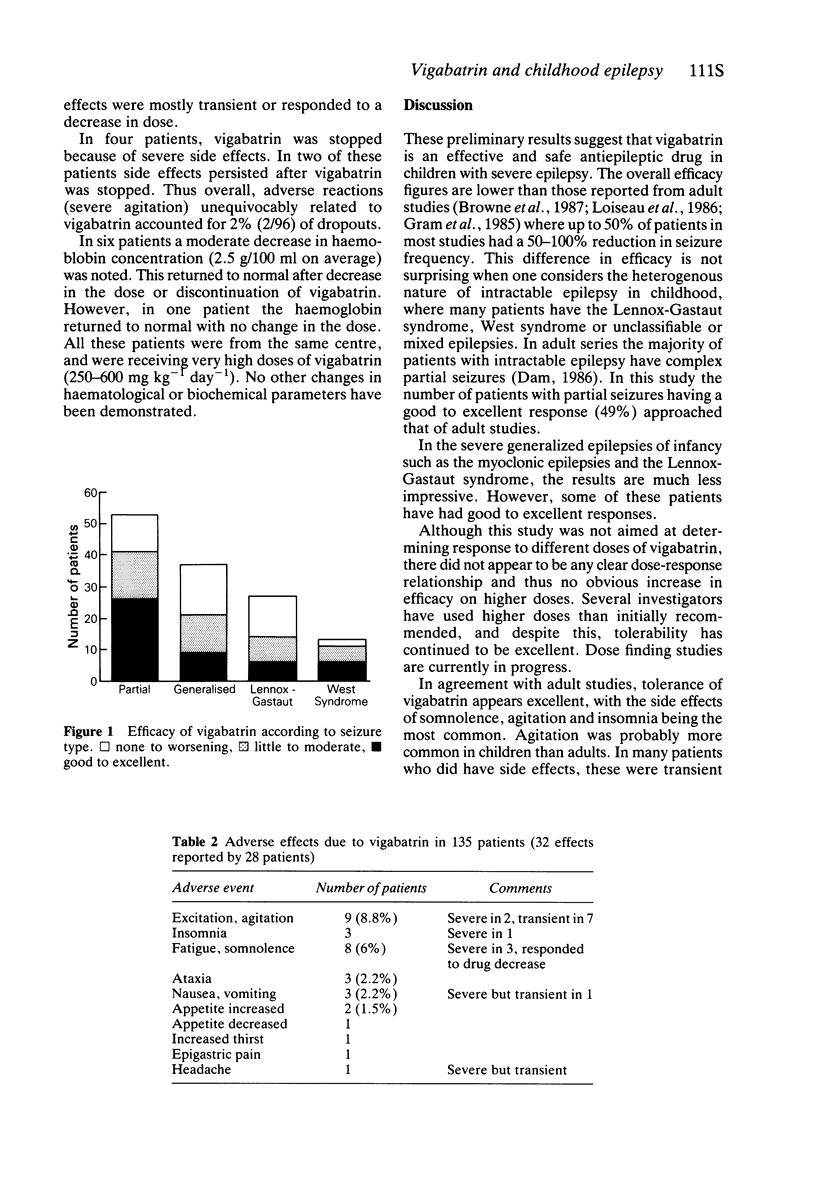
4 A 75% to 100% reduction in seizure frequency was observed in 25% of patients (11 patients became seizure free) and 50 to 75% decrease in a further 13%. Efficacy was better in partial seizures, with good to excellent results in 49% of patients. The use of high doses, above 100 mg kg-1 day-1, was not associated with greater efficacy in this preliminary study.
5 No side effects were reported in 79% of patients. Agitation and insomnia were observed in 8.8% and somnolence in 6%. Other adverse events included ataxia (2.2%), nausea (2.2%) and increased appetite (1%). A moderate and transient decrease in haemoglobin was reported in six patients from the same centre; these patients were all receiving very high doses of vigabatrin (250 to 600 mg kg-1 day-1).
6 Vigabatrin thus appears to be a safe antiepileptic drug that may be effective in the treatment of severe epilepsy in children.
Keywords: intractable epilepsy, children, vigabatrin
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brorson L. O., Wranne L. Long-term prognosis in childhood epilepsy: survival and seizure prognosis. Epilepsia. 1987 Jul-Aug;28(4):324–330. doi: 10.1111/j.1528-1157.1987.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Browne T. R., Mattson R. H., Penry J. K., Smith D. B., Treiman D. M., Wilder B. J., Ben-Menachem E., Napoliello M. J., Sherry K. M., Szabo G. K. Vigabatrin for refractory complex partial seizures: multicenter single-blind study with long-term follow-up. Neurology. 1987 Feb;37(2):184–189. doi: 10.1212/wnl.37.2.184. [DOI] [PubMed] [Google Scholar]
- Gram L., Klosterskov P., Dam M. gamma-Vinyl GABA: a double-blind placebo-controlled trial in partial epilepsy. Ann Neurol. 1985 Mar;17(3):262–266. doi: 10.1002/ana.410170307. [DOI] [PubMed] [Google Scholar]
- Gram L., Larsson O. M., Johnsen A., Schousboe A. Experimental studies of the influence of vigabatrin on the GABA system. Br J Clin Pharmacol. 1989;27 (Suppl 1):13S–17S. doi: 10.1111/j.1365-2125.1989.tb03455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau P., Hardenberg J. P., Pestre M., Guyot M., Schechter P. J., Tell G. P. Double-blind, placebo-controlled study of vigabatrin (gamma-vinyl GABA) in drug-resistant epilepsy. Epilepsia. 1986 Mar-Apr;27(2):115–120. doi: 10.1111/j.1528-1157.1986.tb03512.x. [DOI] [PubMed] [Google Scholar]
- Mumford J. P., Dam M. Meta-analysis of European placebo controlled studies of vigabatrin in drug resistant epilepsy. Br J Clin Pharmacol. 1989;27 (Suppl 1):101S–107S. doi: 10.1111/j.1365-2125.1989.tb03469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter P. J. Clinical pharmacology of vigabatrin. Br J Clin Pharmacol. 1989;27 (Suppl 1):19S–22S. doi: 10.1111/j.1365-2125.1989.tb03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


