Abstract
1 The pharmacokinetics of a single 1 mg dose of cilazapril were determined in six subjects with normal renal function and in 19 uraemic patients with various degrees of renal impairment.
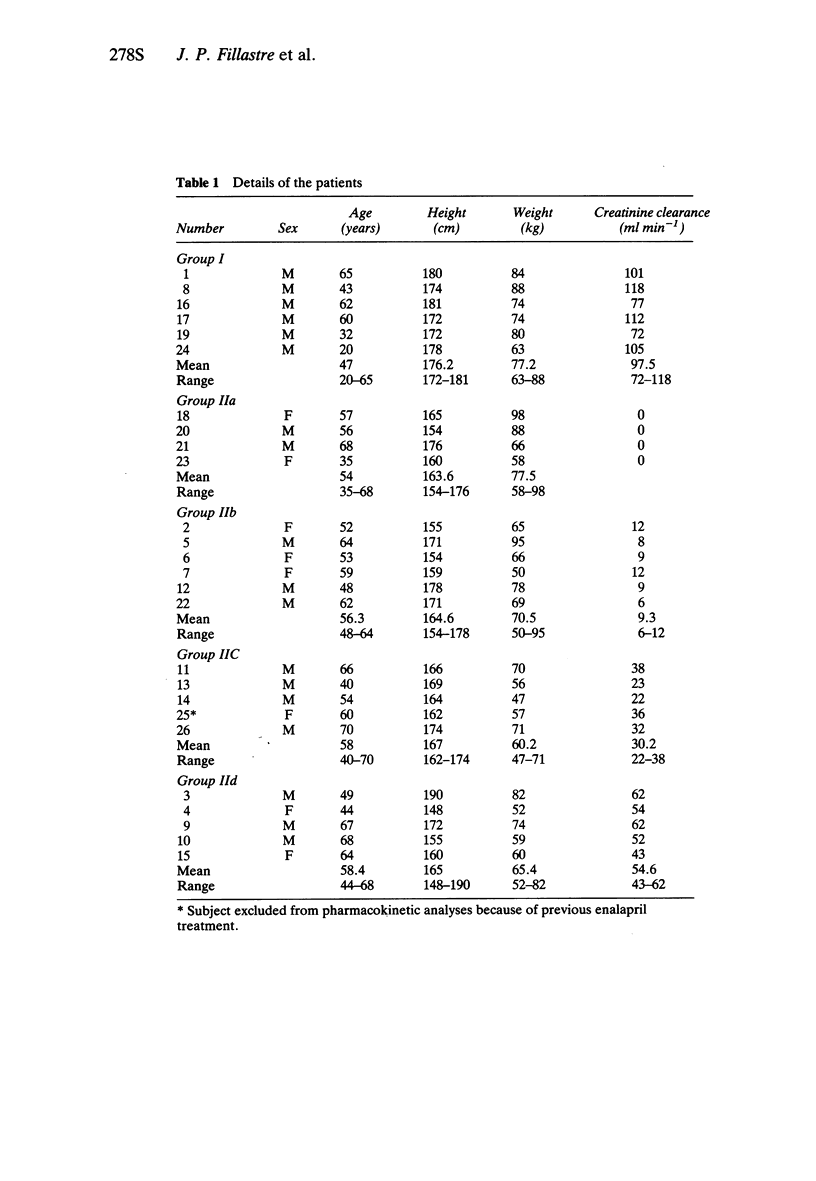
2 Significant decreases in systolic and diastolic blood pressure were noted in all groups of subjects between 2 and 8 h after administration of 1 mg cilazapril.
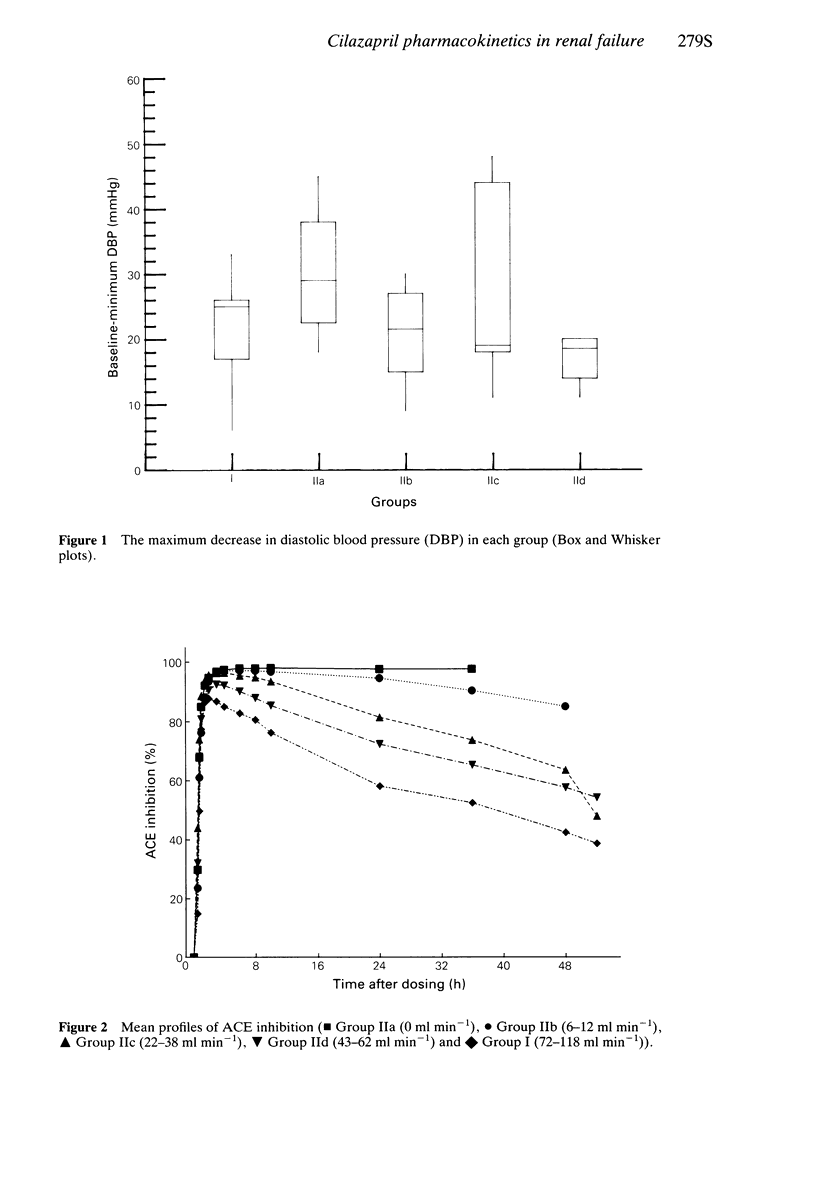
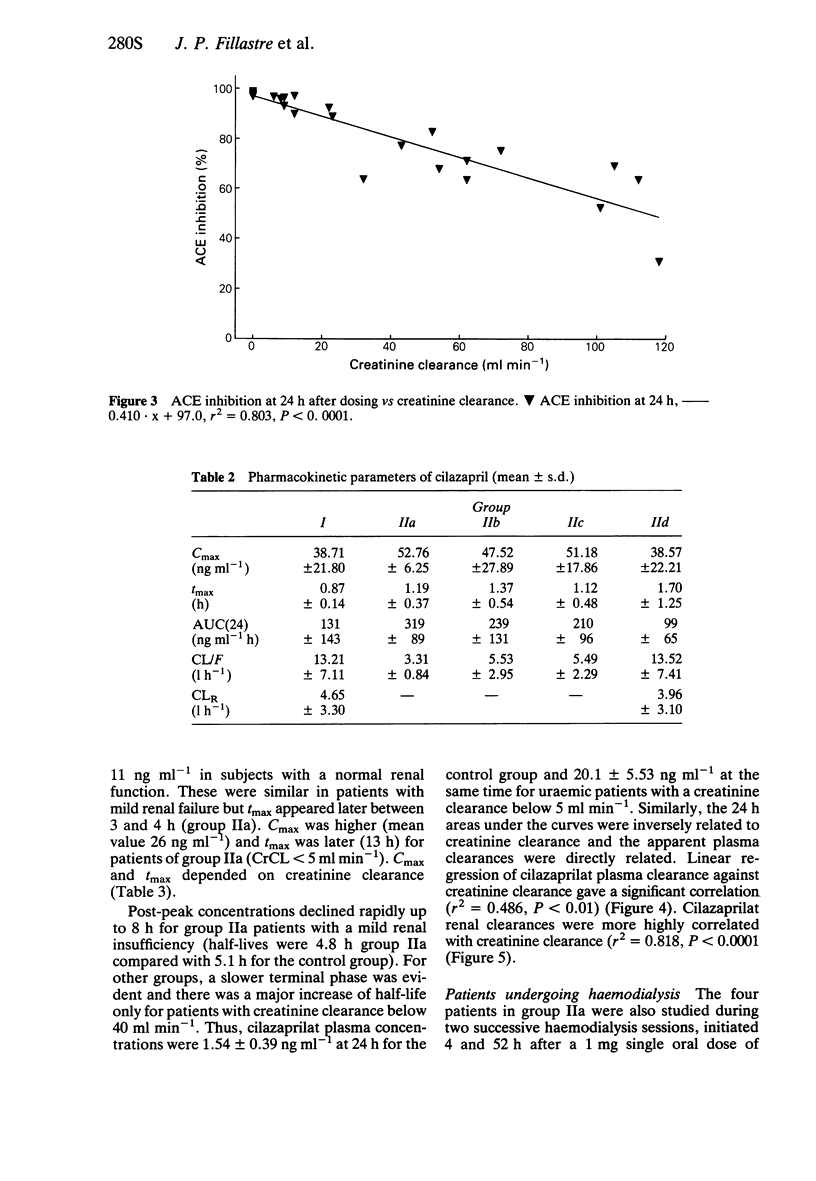
3 There was a significant correlation between ACE inhbition at 24 h and creatinine clearance (CrCL).
4 For cilazapril, Cmax and tmax were independent of creatinine clearance. AUC(24) was inversely related to CrCL and apparent plasma clearance (CL/F) was directly related to CrCL.
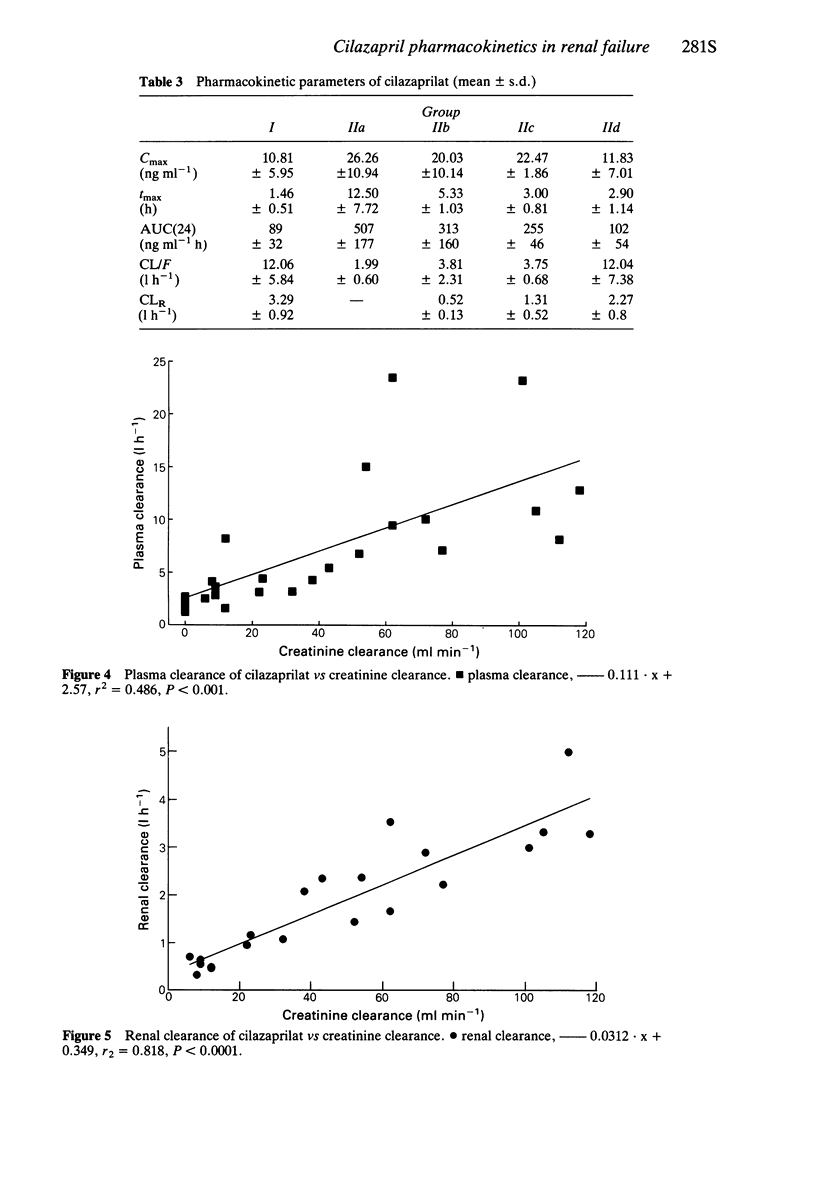
5 For cilazaprilat, Cmax and tmax were related to creatinine clearance. AUC(24) was inversely related to CrCl and apparent plasma clearance (CL/F) was directly related to CrCL.
6 Dialysis clearance was approximately 21 h-1 for cilazapril and for cilazaprilat.
7 The effects of renal impairment on cilazapril and cilazaprilat kinetics were similar to those observed for other inhibitors of angiotensin-converting enzyme such as captopril, enalapril and lisinopril.
8 It may be necessary to modify doses of cilazapril for the treatment of essential hypertension in uraemic patients. When creatinine clearance was below 15 ml min-1 cilazaprilat concentrations were increased, half-lives were prolonged and ACE inhibition remained above 90% for at least 24 h. A reduced dosage is indicated for these patients.
9 In patients requiring haemodialysis, maintenance doses of 0.5 mg given after each haemodialysis session are sufficient.
Keywords: cilazapril, blood pressure, renal impairment, angiotensin converting enzyme inhibition, creatinine clearance
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Francis R. J., Brown A. N., Kler L., Fasanella d'Amore T., Nussberger J., Waeber B., Brunner H. R. Pharmacokinetics of the converting enzyme inhibitor cilazapril in normal volunteers and the relationship to enzyme inhibition: development of a mathematical model. J Cardiovasc Pharmacol. 1987 Jan;9(1):32–38. [PubMed] [Google Scholar]
- Kelly J. G., Doyle G., Donohue J., Laher M., Vandenburg M. J., Currie W. J., Cooper W. D. Pharmacokinetics of enalapril in normal subjects and patients with renal impairment. Br J Clin Pharmacol. 1986 Jan;21(1):63–69. doi: 10.1111/j.1365-2125.1986.tb02823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal D. T., Irvin J. D., Merrill D., Saris S., Ulm E., Goldstein S., Hichens M., Klein L., Till A., Harris K. The effect of renal function on enalapril kinetics. Clin Pharmacol Ther. 1985 Dec;38(6):661–666. doi: 10.1038/clpt.1985.242. [DOI] [PubMed] [Google Scholar]
- Shionoiri H., Gotoh E., Takagi N., Takeda K., Yabana M., Kaneko Y. Antihypertensive effects and pharmacokinetics of single and consecutive doses of cilazapril in hypertensive patients with normal and impaired renal function. J Cardiovasc Pharmacol. 1988 Feb;11(2):242–249. [PubMed] [Google Scholar]
- Whitehead E. M., Walters G. E., Williams P. E., Johnston G. D. Pharmacokinetics of intravenous cilazaprilat in normal volunteers. Br J Clin Pharmacol. 1989 Jun;27(6):873–875. doi: 10.1111/j.1365-2125.1989.tb03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik B. A., Geyskes G. G., Boer P. Lisinopril in hypertensive patients with and without renal failure. Eur J Clin Pharmacol. 1987;32(1):11–16. doi: 10.1007/BF00609951. [DOI] [PubMed] [Google Scholar]


