Abstract
1. Five healthy volunteers received, in a randomised crossover design study, a 155 mg oral tablet and an intravenous infusion of 155 mg racemic hydroxychloroquine (200 mg hydroxychloroquine sulphate) to assess the bioavailability of the commercially available tablet (Plaquenil, Winthrop Laboratories, Australia). 2. The terminal elimination half-life of hydroxychloroquine is more than 40 days, thus blood and urine samples were collected for 5 months following each dose to characterise adequately the terminal elimination phase and obtain accurate estimates of the areas under the concentration-time curves. 3. The mean (+/- s.d.) fraction of the oral dose absorbed, estimated from the blood and urine data, was 0.74 (+/- 0.13). A wide range of estimates of the fraction of the oral dose absorbed was calculated from the plasma data (0.41 - 1.53), reflecting the difficulties of accurate measurement of hydroxychloroquine in plasma. 4. A period of 6 months is required to achieve 96% of steady-state levels of hydroxychloroquine with the usual once daily, oral dosage regimen. Pharmacokinetic factors may thus be partly responsible for the delayed action of the drug in rheumatic conditions. 5. Haemodialysis will not aid in the case of oral overdose with hydroxychloroquine. Although the proportionate increase in clearance may be large, the increase in the fraction of the dose excreted will be negligible. The extensive sequestration of the drug by tissues limits effectiveness of haemodialysis.
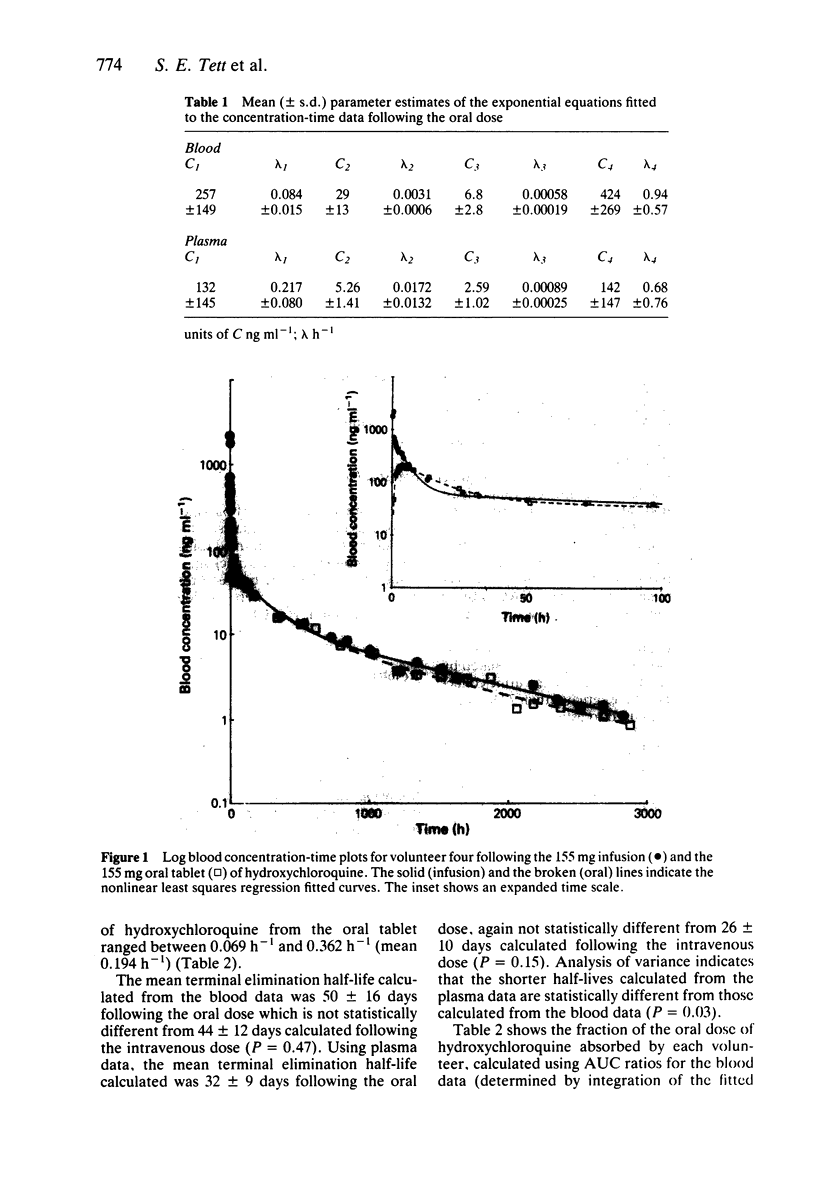
Full text
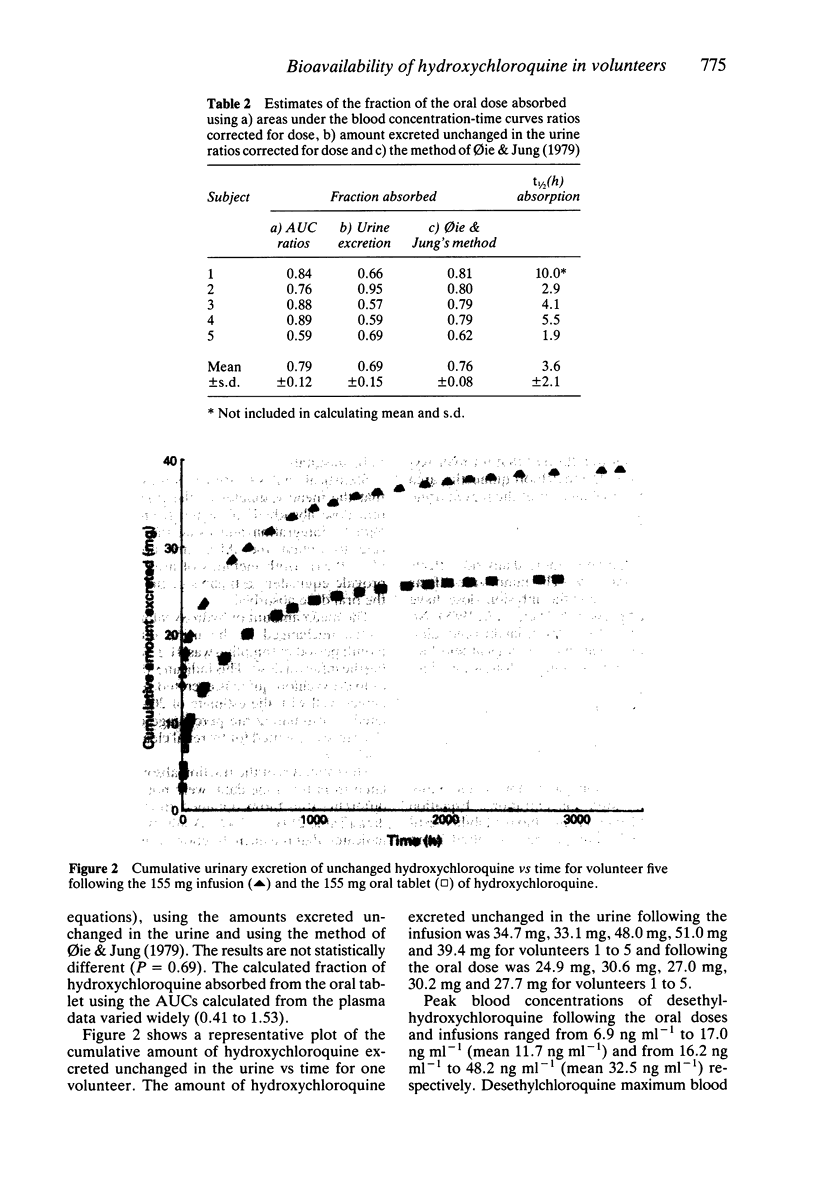
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. M., Yocum D. E., Bell C. L. Hydroxychloroquine in the treatment of rheumatoid arthritis. Am J Med. 1983 Aug;75(2):321–326. doi: 10.1016/0002-9343(83)91211-1. [DOI] [PubMed] [Google Scholar]
- Akintonwa A., Odutola T. A., Edeki T., Mabadeje A. F. Hemodialysis clearance of chloroquine in uremic patients. Ther Drug Monit. 1986;8(3):285–287. doi: 10.1097/00007691-198609000-00008. [DOI] [PubMed] [Google Scholar]
- Bergqvist Y., Domeij-Nyberg B. Distribution of chloroquine and its metabolite desethyl-chloroquine in human blood cells and its implication for the quantitative determination of these compounds in serum and plasma. J Chromatogr. 1983 Jan 14;272(1):137–148. doi: 10.1016/s0378-4347(00)86110-1. [DOI] [PubMed] [Google Scholar]
- CANN H. M., VERHULST H. L. Fatal acute chloroquine poisoning in children. Pediatrics. 1961 Jan;27:95–102. [PubMed] [Google Scholar]
- Cutler D. J. Numerical deconvolution by least squares: use of prescribed input functions. J Pharmacokinet Biopharm. 1978 Jun;6(3):227–241. doi: 10.1007/BF01312264. [DOI] [PubMed] [Google Scholar]
- Di Maio V. J., Henry L. D. Chloroquine poisoning. South Med J. 1974 Sep;67(9):1031–1035. doi: 10.1097/00007611-197409000-00007. [DOI] [PubMed] [Google Scholar]
- Dwosh I. L., Stein H. B., Urowitz M. B., Smythe H. A., Hunter T., Ogryzlo M. A. Azathioprine in early rheumatoid arthritis. Comparison with gold and chloroquine. Arthritis Rheum. 1977 Mar;20(2):685–692. doi: 10.1002/art.1780200208. [DOI] [PubMed] [Google Scholar]
- French J. K., Hurst N. P., O'Donnell M. L., Betts W. H. Uptake of chloroquine and hydroxychloroquine by human blood leucocytes in vitro: relation to cellular concentrations during antirheumatic therapy. Ann Rheum Dis. 1987 Jan;46(1):42–45. doi: 10.1136/ard.46.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON E. B., SCOTT J. T. Hydroxychloroquine sulfate ("plaguenil") in treatment of rheumatoid arthritis. Arthritis Rheum. 1962 Oct;5:502–512. doi: 10.1002/art.1780050507. [DOI] [PubMed] [Google Scholar]
- Husain Z., Runge L. A. Treatment complications of rheumatoid arthritis with gold, hydroxychloroquine, D-penicillamine, and levamisole. J Rheumatol. 1980 Nov-Dec;7(6):825–830. [PubMed] [Google Scholar]
- KERSLEY G. D., PALIN A. G. Amodiaquine and hydroxychloroquine in rheumatoid arthritis. Lancet. 1959 Nov 21;2(7108):886–888. doi: 10.1016/s0140-6736(59)90808-6. [DOI] [PubMed] [Google Scholar]
- Miller D. R., Fiechtner J. J., Carpenter J. R., Brown R. R., Stroshane R. M., Stecher V. J. Plasma hydroxychloroquine concentrations and efficacy in rheumatoid arthritis. Arthritis Rheum. 1987 May;30(5):567–571. doi: 10.1002/art.1780300512. [DOI] [PubMed] [Google Scholar]
- Oie S., Jung D. Bioavailability under variable renal clearance conditions. J Pharm Sci. 1979 Jan;68(1):128–129. doi: 10.1002/jps.2600680148. [DOI] [PubMed] [Google Scholar]
- Paulus H. E. An overview of benefit/risk of disease modifying treatment of rheumatoid arthritis as of today. Ann Rheum Dis. 1982;41 (Suppl 1):26–29. doi: 10.1136/ard.41.suppl_1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier D., Gibaldi M. General derivation of the equation for time to reach a certain fraction of steady state. J Pharm Sci. 1982 Apr;71(4):474–475. doi: 10.1002/jps.2600710432. [DOI] [PubMed] [Google Scholar]
- Raghoebar M., Peeters P. A., van den Berg W. B., van Ginneken C. A. Mechanisms of cell association of chloroquine to leucocytes. J Pharmacol Exp Ther. 1986 Jul;238(1):302–306. [PubMed] [Google Scholar]
- Richter J. A., Runge L. A., Pinals R. S., Oates R. P. Analysis of treatment terminations with gold and antimalarial compounds in rheumatoid arthritis. J Rheumatol. 1980 Mar-Apr;7(2):153–159. [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J. Apparent dose-dependence of chloroquine pharmacokinetics due to limited assay sensitivity and short sampling times. Eur J Clin Pharmacol. 1987;31(6):729–731. doi: 10.1007/BF00541305. [DOI] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J., Brown K. F. High-performance liquid chromatographic assay for hydroxychloroquine and metabolites in blood and plasma, using a stationary phase of poly(styrene divinylbenzene) and a mobile phase at pH 11, with fluorimetric detection. J Chromatogr. 1985 Nov 8;344:241–248. doi: 10.1016/s0378-4347(00)82024-1. [DOI] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J., Brown K. F. Removal of an endogenous fluorescent compound from urine to allow quantitation of low concentrations of hydroxychloroquine and metabolites by high-performance liquid chromatography. J Chromatogr. 1986 Nov 28;383(1):236–238. doi: 10.1016/s0378-4347(00)83468-4. [DOI] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J., Day R. O., Brown K. F. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1988 Sep;26(3):303–313. doi: 10.1111/j.1365-2125.1988.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]



