Abstract
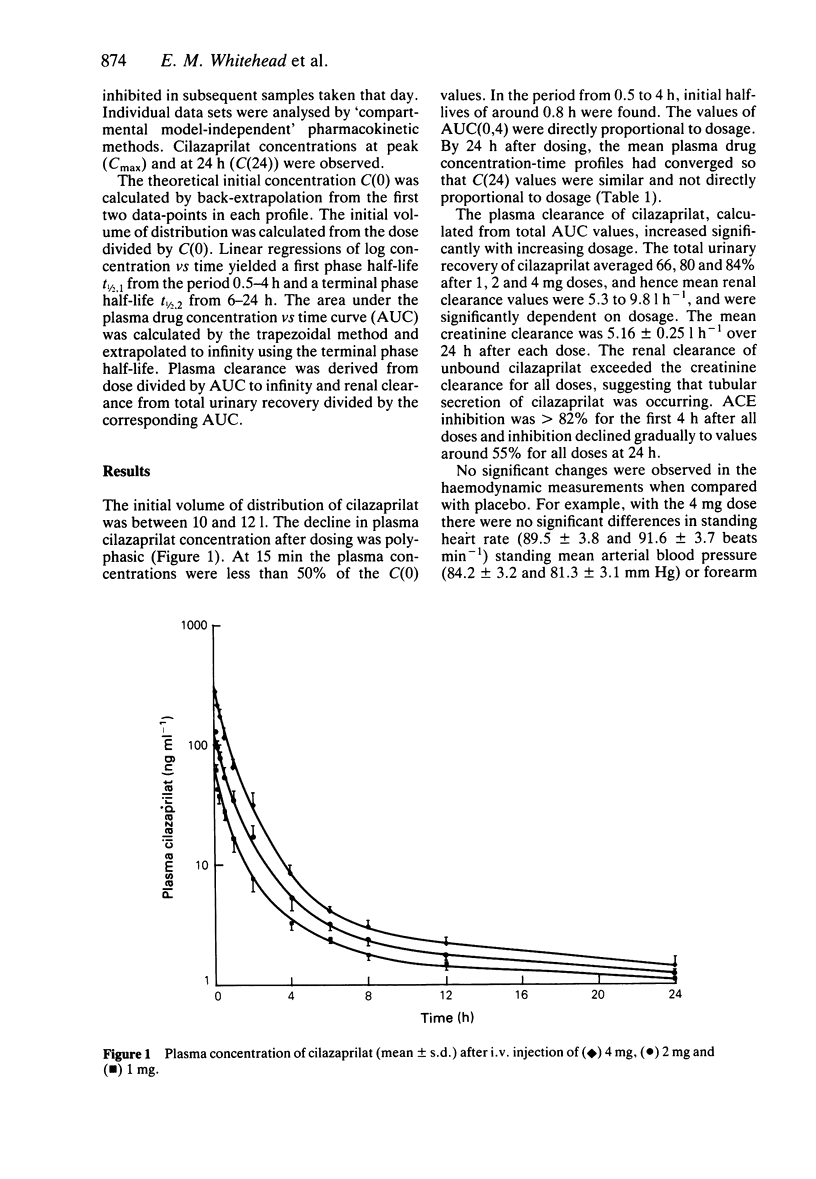
The pharmacokinetics of 1 mg, 2 mg and 4 mg of cilazaprilat administered intravenously were determined in a group of eight volunteers. The fall in plasma concentration was polyphasic. Elimination was predominantly by renal excretion of the unchanged drug. The mean renal clearance values following 1 mg, 2 mg and 4 mg doses were 5.3 +/- 0.5, 8.1 +/- 0.5, and 9.8 +/- 0.5 l h-1 and plasma clearances were 7.8 +/- 0.5, 10.4 +/- 0.5 and 11.8 +/- 0.6 l h-1, respectively. Thus, plasma and renal clearances were dose dependent. ACE inhibition was greater than 82% for the first 4 h and about 55% at 24 h, after all three doses. There were no significant haemodynamic effects at any dose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duchin K. L., Singhvi S. M., Willard D. A., Migdalof B. H., McKinstry D. N. Captopril kinetics. Clin Pharmacol Ther. 1982 Apr;31(4):452–458. doi: 10.1038/clpt.1982.59. [DOI] [PubMed] [Google Scholar]
- Fasanella d'Amore T., Bussien J. P., Nussberger J., Waeber B., Turini G. A., Brunner H. R., Kler L., Francis R. J. Effects of single doses of the converting enzyme inhibitor cilazapril in normal volunteers. J Cardiovasc Pharmacol. 1987 Jan;9(1):26–31. [PubMed] [Google Scholar]
- Francis R. J., Brown A. N., Kler L., Fasanella d'Amore T., Nussberger J., Waeber B., Brunner H. R. Pharmacokinetics of the converting enzyme inhibitor cilazapril in normal volunteers and the relationship to enzyme inhibition: development of a mathematical model. J Cardiovasc Pharmacol. 1987 Jan;9(1):32–38. [PubMed] [Google Scholar]
- Natoff I. L., Nixon J. S., Francis R. J., Klevans L. R., Brewster M., Budd J., Patel A. T., Wenger J., Worth E. Biological properties of the angiotensin-converting enzyme inhibitor cilazapril. J Cardiovasc Pharmacol. 1985 May-Jun;7(3):569–580. doi: 10.1097/00005344-198505000-00025. [DOI] [PubMed] [Google Scholar]
- Singhvi S. M., Duchin K. L., Morrison R. A., Willard D. A., Everett D. W., Frantz M. Disposition of fosinopril sodium in healthy subjects. Br J Clin Pharmacol. 1988 Jan;25(1):9–15. doi: 10.1111/j.1365-2125.1988.tb03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm E. H., Hichens M., Gomez H. J., Till A. E., Hand E., Vassil T. C., Biollaz J., Brunner H. R., Schelling J. L. Enalapril maleate and a lysine analogue (MK-521): disposition in man. Br J Clin Pharmacol. 1982 Sep;14(3):357–362. doi: 10.1111/j.1365-2125.1982.tb01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


