Abstract
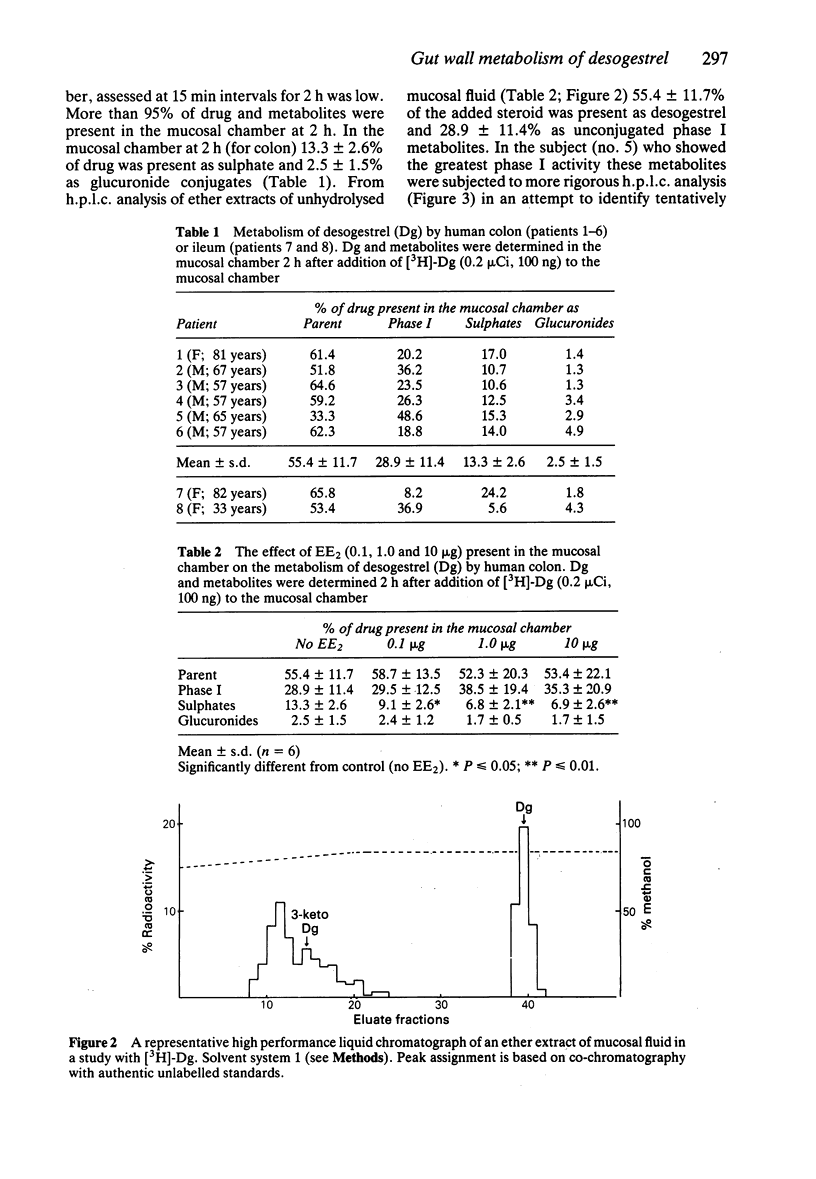
1. The intestinal mucosal metabolism of the progestogen oral contraceptive desogestrel (Dg) has been studied in vitro using the Ussing chamber technique. Histologically normal ileum or colon was obtained from eight patients undergoing various resections. The mucosal sheets were mounted between two perspex chambers. 2. Two hours after addition of [3H]-Dg (0.2 microCi; 100 ng) to the mucosal chamber, more than 90% of the steroid was present in that chamber. In studies with colon, metabolite analysis showed that 55.4 +/- 11.7% (mean +/- s.d.; n = 6) of drug present was Dg, 28.9 +/- 11.4% as unconjugated Phase I metabolites, 13.3 +/- 2.6% as sulphate conjugates and 2.5 +/- 1.5% as glucuronide conjugates. 3. By co-chromatography with authentic metabolites and mass spectrometry, it was shown that 3-keto desogestrel is formed in the mucosa. This is the active metabolite of desogestrel. A large peak of radioactivity did not co-chromatograph with any known metabolites and has been tentatively identified as ring hydroxylated products of 3-keto desogestrel. 4. The effect of the synthetic oestrogen ethinyloestradiol (EE2) on the metabolite profile of Dg was studied. In the presence of increasing concentrations of EE2 (100 ng, 1 and 10 micrograms), there was competition for sulphation such that the sulphate fraction decreased by 32, 49 and 48% respectively. 5. The results of this study indicate substantial first pass metabolism of desogestrel by the gut mucosa with evidence for the formation of the active metabolite. The extent of phase I metabolism is unusual.
Full text
PDF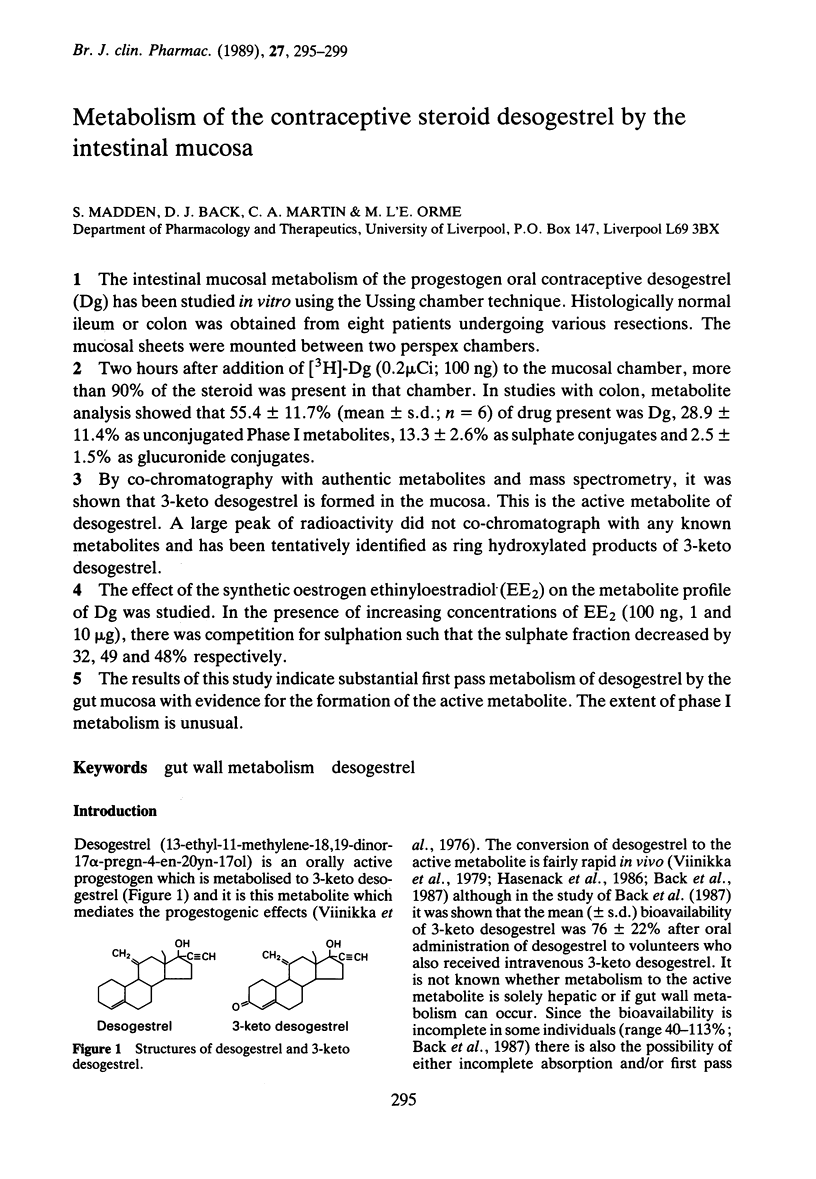



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back D. J., Breckenridge A. M., MacIver M., Orme M., Purba H. S., Rowe P. H., Taylor I. The gut wall metabolism of ethinyloestradiol and its contribution to the pre-systemic metabolism of ethinyloestradiol in humans. Br J Clin Pharmacol. 1982 Mar;13(3):325–330. doi: 10.1111/j.1365-2125.1982.tb01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back D. J., Grimmer S. F., Shenoy N., Orme M. L. Plasma concentrations of 3-keto-desogestrel after oral administration of desogestrel and intravenous administration of 3-keto-desogestrel. Contraception. 1987 Jun;35(6):619–626. doi: 10.1016/s0010-7824(87)80021-5. [DOI] [PubMed] [Google Scholar]
- Back D. J., Rogers S. M. Review: first-pass metabolism by the gastrointestinal mucosa. Aliment Pharmacol Ther. 1987 Oct;1(5):339–357. doi: 10.1111/j.1365-2036.1987.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Grady G. F., Duhamel R. C., Moore E. W. Active transport of sodium by human colon in vitro. Gastroenterology. 1970 Oct;59(4):583–588. [PubMed] [Google Scholar]
- Hartmann F., Gruenke L. D., Craig J. C., Bissell D. M. Chlorpromazine metabolism in extracts of liver and small intestine from guinea pig and from man. Drug Metab Dispos. 1983 May-Jun;11(3):244–248. [PubMed] [Google Scholar]
- Hasenack H. G., Bosch A. M., Kär K. Serum levels of 3-keto-desogestrel after oral administration of desogestrel and 3-keto-desogestrel. Contraception. 1986 Jun;33(6):591–596. doi: 10.1016/0010-7824(86)90047-8. [DOI] [PubMed] [Google Scholar]
- Pacifici G. M., Back D. J. Sulphation and glucuronidation of ethinyloestradiol in human liver in vitro. J Steroid Biochem. 1988 Sep;31(3):345–349. doi: 10.1016/0022-4731(88)90360-3. [DOI] [PubMed] [Google Scholar]
- Rogers S. M., Back D. J., Orme M. L. Intestinal metabolism of ethinyloestradiol and paracetamol in vitro: studies using Ussing chambers. Br J Clin Pharmacol. 1987 Jun;23(6):727–734. doi: 10.1111/j.1365-2125.1987.tb03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viinikka L., Ylikorkala O., Nummi S., Virkkunen P., Ranta T., Alapiessa U., Vihko R. Biological effects of a new and potent progestagen. A clinical study. Acta Endocrinol (Copenh) 1976 Oct;83(2):429–438. doi: 10.1530/acta.0.0830429. [DOI] [PubMed] [Google Scholar]
- Viinikka L., Ylikorkala O., Vihko R., Wijnand H. P., Booij M., van der Veen F. Metabolism of a new synthetic progestagen, Org 2969, in female volunteers. Pharmacokinetics after an oral dose. Eur J Clin Pharmacol. 1979 Jun 12;15(5):349–355. doi: 10.1007/BF00558439. [DOI] [PubMed] [Google Scholar]


