Abstract
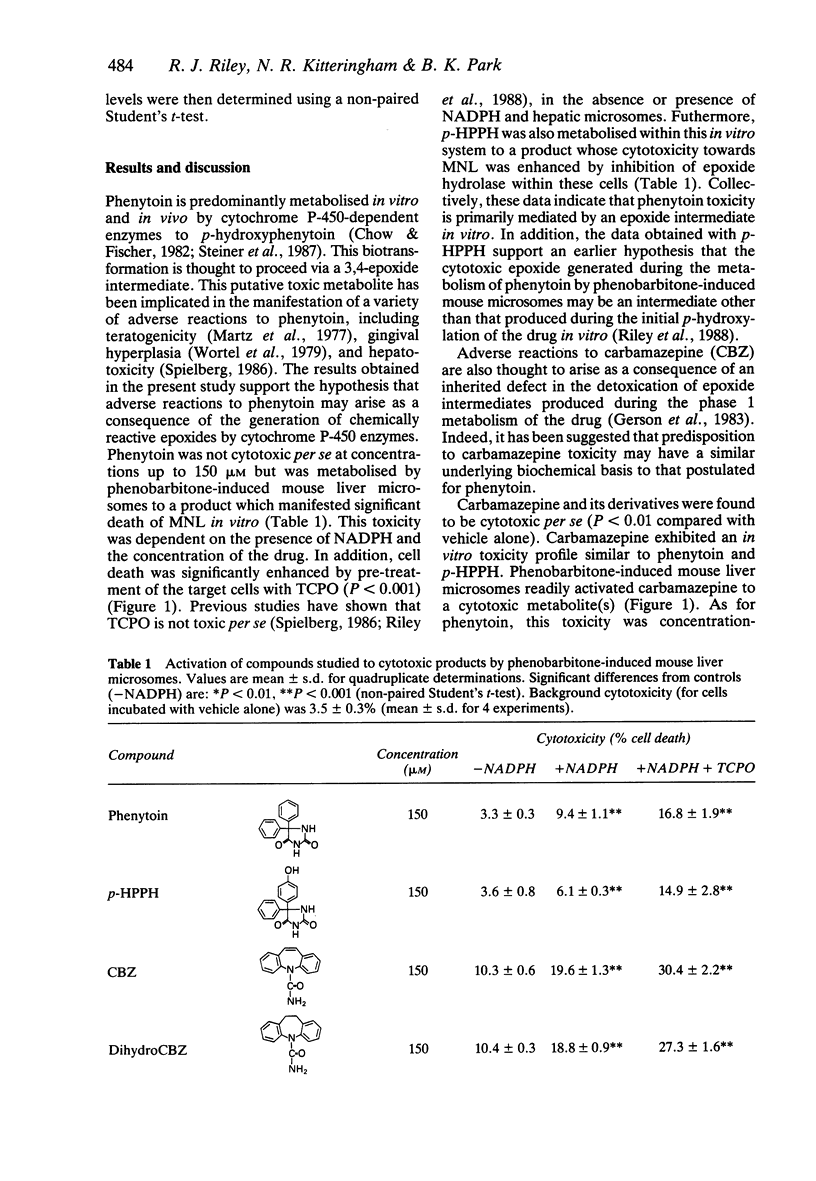
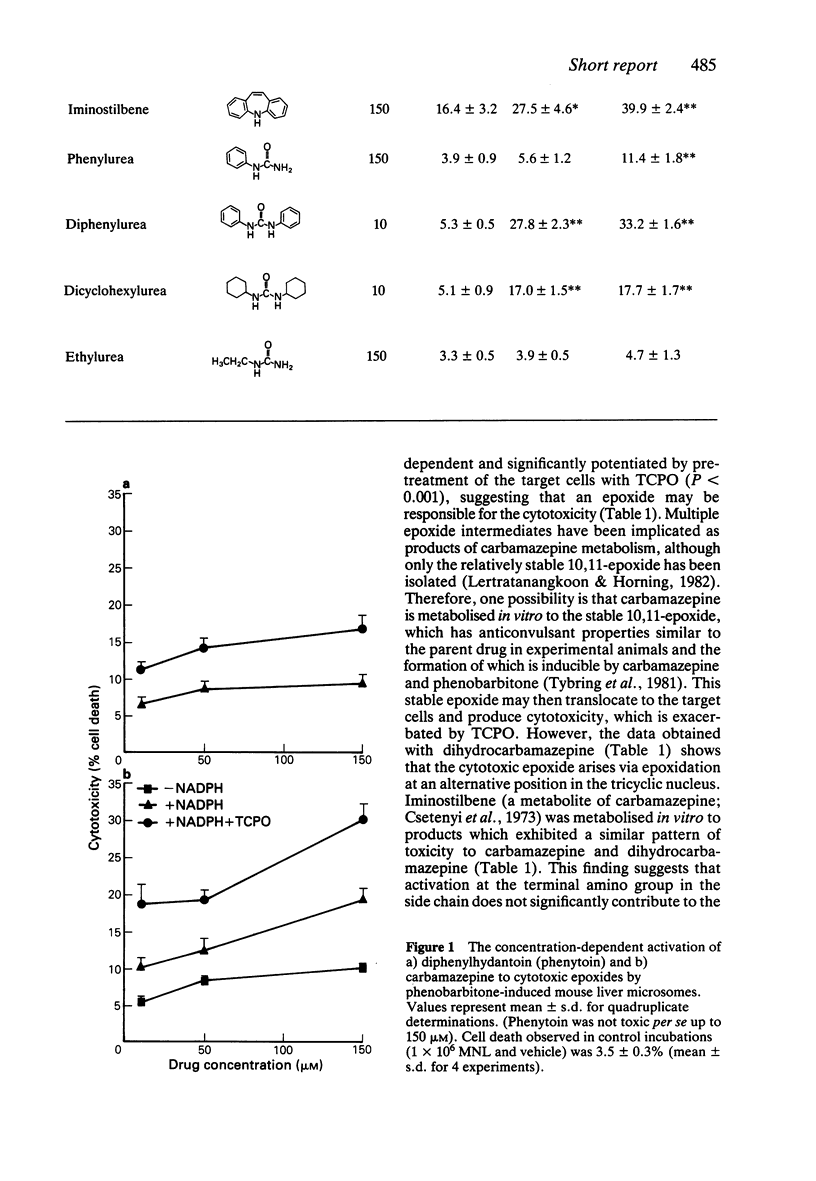
The formation of cytotoxic metabolites from the anticonvulsants phenytoin and carbamazepine was investigated in vitro using a hepatic microsomal enzyme system and human mononuclear leucocytes as target cells. Both drugs were metabolised to cytotoxic products. In order to assess the structural requirements for this bioactivation, a series of structurally related compounds was investigated. It was found that molecules which contain either an amide function or an aryl ring may undergo activation in vitro, but only the metabolism-dependent toxicity of the latter is potentiated by pre-treatment of the target cells with an epoxide hydrolase inhibitor. Taken collectively, these data are consistent with the concept that reactive epoxide metabolites of both phenytoin and carbamazepine may produce toxicity in individuals with an inherited deficiency in epoxide hydrolase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bøyum A. Separation of lymphocytes, granulocytes, and monocytes from human blood using iodinated density gradient media. Methods Enzymol. 1984;108:88–102. doi: 10.1016/s0076-6879(84)08076-9. [DOI] [PubMed] [Google Scholar]
- Chow S. A., Fischer L. J. Phenytoin metabolism in mice. Drug Metab Dispos. 1982 Mar-Apr;10(2):156–160. [PubMed] [Google Scholar]
- Csetenyi J., Baker K. M., Frigerio A., Morselli P. L. Iminostilbene--a metabolite of carbamazepine isolated from rat urine. J Pharm Pharmacol. 1973 Apr;25(4):340–341. doi: 10.1111/j.2042-7158.1973.tb10021.x. [DOI] [PubMed] [Google Scholar]
- Gerson W. T., Fine D. G., Spielberg S. P., Sensenbrenner L. L. Anticonvulsant-induced aplastic anemia: increased susceptibility to toxic drug metabolites in vitro. Blood. 1983 May;61(5):889–893. [PubMed] [Google Scholar]
- Grossman S. J., Jollow D. J. Role of dapsone hydroxylamine in dapsone-induced hemolytic anemia. J Pharmacol Exp Ther. 1988 Jan;244(1):118–125. [PubMed] [Google Scholar]
- Guengerich F. P., Liebler D. C. Enzymatic activation of chemicals to toxic metabolites. Crit Rev Toxicol. 1985;14(3):259–307. doi: 10.3109/10408448509037460. [DOI] [PubMed] [Google Scholar]
- Holme J. A., Søderlund E. J. Species differences in the cytotoxic and genotoxic effects of 2-acetylaminofluorene and its primary metabolites 2-aminofluorene and N-OH-2-acetylaminofluorene. Carcinogenesis. 1985 Mar;6(3):421–425. doi: 10.1093/carcin/6.3.421. [DOI] [PubMed] [Google Scholar]
- Jaspan J. Pharmacological inhibition of aldose reductase in human diabetic neuropathy. Drugs. 1986;32 (Suppl 2):23–29. doi: 10.2165/00003495-198600322-00007. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W. Arene oxides: a new aspect of drug metabolism. Science. 1974 Aug 16;185(4151):573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lertratanangkoon K., Horning M. G. Metabolism of carbamazepine. Drug Metab Dispos. 1982 Jan-Feb;10(1):1–10. [PubMed] [Google Scholar]
- Maggs J. L., Grabowski P. S., Park B. K. Drug protein conjugates--III. Inhibition of the irreversible binding of ethinylestradiol to rat liver microsomal protein by mixed-function oxidase inhibitors, ascorbic acid and thiols. J Steroid Biochem. 1983 Sep;19(3):1273–1278. doi: 10.1016/0022-4731(83)90150-4. [DOI] [PubMed] [Google Scholar]
- Martz F., Failinger C., 3rd, Blake D. A. Phenytoin teratogenesis: correlation between embryopathic effect and covalent binding of putative arene oxide metabolite in gestational tissue. J Pharmacol Exp Ther. 1977 Oct;203(1):231–239. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Park B. K., Coleman J. W., Kitteringham N. R. Drug disposition and drug hypersensitivity. Biochem Pharmacol. 1987 Mar 1;36(5):581–590. [PubMed] [Google Scholar]
- Purba H. S., Maggs J. L., Orme M. L., Back D. J., Park B. K. The metabolism of 17 alpha-ethinyloestradiol by human liver microsomes: formation of catechol and chemically reactive metabolites. Br J Clin Pharmacol. 1987 Apr;23(4):447–453. doi: 10.1111/j.1365-2125.1987.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Maggs J. L., Lambert C., Kitteringham N. R., Park B. K. An in vitro study of the microsomal metabolism and cellular toxicity of phenytoin, sorbinil and mianserin. Br J Clin Pharmacol. 1988 Nov;26(5):577–588. doi: 10.1111/j.1365-2125.1988.tb05298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg S. P. In vitro analysis of idiosyncratic drug reactions. Clin Biochem. 1986 Apr;19(2):142–144. doi: 10.1016/s0009-9120(86)80061-3. [DOI] [PubMed] [Google Scholar]
- Steiner E., Alván G., Garle M., Maguire J. H., Lind M., Nilson S. O., Tomson T., McClanahan J. S., Sjöqvist F. The debrisoquin hydroxylation phenotype does not predict the metabolism of phenytoin. Clin Pharmacol Ther. 1987 Sep;42(3):326–333. doi: 10.1038/clpt.1987.156. [DOI] [PubMed] [Google Scholar]
- Tybring G., von Bahr C., Bertilsson L., Collste H., Glaumann H., Solbrand M. Metabolism of carbamazepine and its epoxide metabolite in human and rat liver in vitro. Drug Metab Dispos. 1981 Nov-Dec;9(6):561–564. [PubMed] [Google Scholar]
- Uetrecht J., Zahid N. N-chlorination of phenytoin by myeloperoxidase to a reactive metabolite. Chem Res Toxicol. 1988 May-Jun;1(3):148–151. doi: 10.1021/tx00003a004. [DOI] [PubMed] [Google Scholar]
- Uetrecht J., Zahid N., Shear N. H., Biggar W. D. Metabolism of dapsone to a hydroxylamine by human neutrophils and mononuclear cells. J Pharmacol Exp Ther. 1988 Apr;245(1):274–279. [PubMed] [Google Scholar]
- Wortel J. P., Hefferren J. J., Rao G. S. Metabolic activation and covalent binding of phenytoin in the rat gingiva. J Periodontal Res. 1979 Mar;14(2):178–181. doi: 10.1111/j.1600-0765.1979.tb00789.x. [DOI] [PubMed] [Google Scholar]


