Abstract
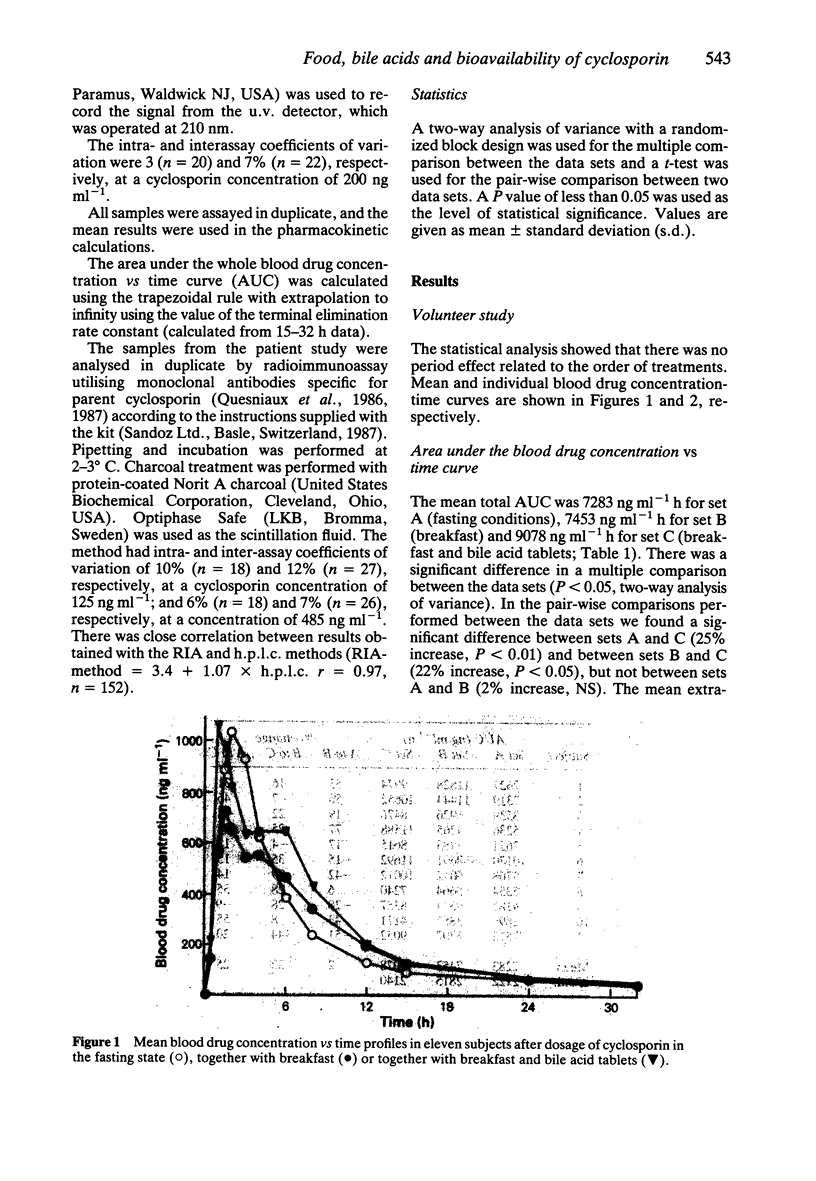
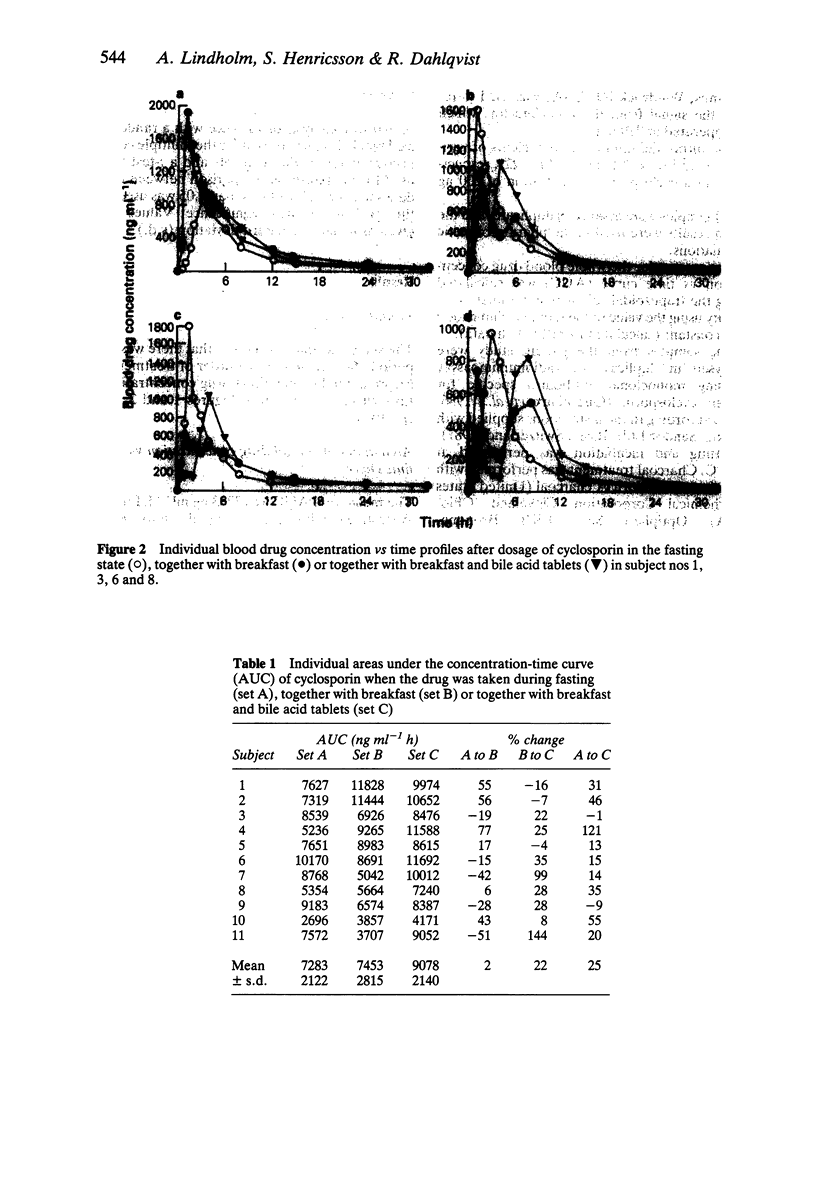
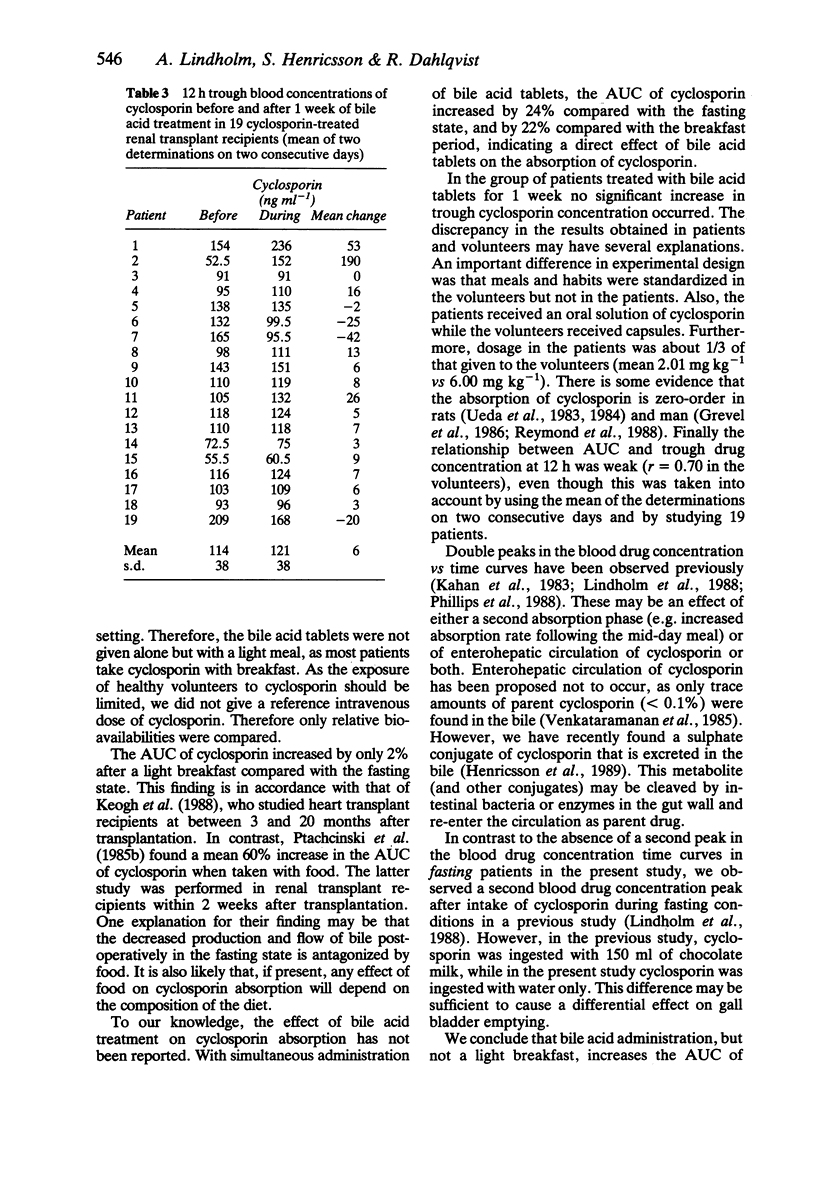
1. The relative bioavailability of cyclosporin was studied in 11 healthy volunteers after single oral capsule doses of cyclosporin on three separate occasions; fasting, with breakfast and with breakfast together with bile acid tablets (400 mg of cholic acid and 100 mg of dehydrocholic acid). 2. There was a significant increase in the area under the blood concentration vs time curve (AUC) of cyclosporin when the drug was taken together with breakfast and bile acid tablets (9078 ng ml-1 h) as compared with breakfast alone (7453 ng ml-1 h, P less than 0.05) or fasting conditions (7283 ng ml-1 h, P less than 0.01). 3. A blood drug concentration vs time curve displaying two peaks was present in 9/11 subjects when cyclosporin was taken with breakfast or with breakfast and bile acid tablets, but only one peak was present when cyclosporin was taken during fasting, suggesting an enterohepatic circulation of cyclosporin or a second absorption phase after the meal. 4. In a separate study, 12 h trough blood cyclosporin concentrations were measured before and after 1 week of bile acid treatment in 19 clinically stable, out-patient transplant recipients who were treated with oral cyclosporin solution (mean dose 2.0 mg kg-1 twice daily). The administration of cyclosporin was not standardized with regard to food intake. There was no significant difference in the blood concentrations of cyclosporin before and after bile acid treatment (114 +/- 38 ng ml-1 vs 121 +/- 38 ng ml-1).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W., Iwatsuki S., Shaw B. W., Jr, Starzl T. E. Cyclosporine monitoring in liver transplant patients. Transplantation. 1985 Mar;39(3):338–338. [PubMed] [Google Scholar]
- Bertault-Pérès P., Maraninchi D., Carcassonne Y., Cano J. P., Barbet J. Clinical pharmacokinetics of ciclosporin A in bone marrow transplantation patients. Cancer Chemother Pharmacol. 1985;15(1):76–81. doi: 10.1007/BF00257300. [DOI] [PubMed] [Google Scholar]
- Burckart G. J., Venkataramanan R., Ptachcinski R. J., Starzl T. E., Gartner J. C., Jr, Zitelli B. J., Malatack J. J., Shaw B. W., Iwatsuki S., Van Thiel D. H. Cyclosporine absorption following orthotopic liver transplantation. J Clin Pharmacol. 1986 Nov-Dec;26(8):647–651. doi: 10.1002/j.1552-4604.1986.tb02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericzon B. G., Todo S., Lynch S., Kam I., Ptachcinski R. J., Burckart G. J., Van Thiel D. H., Starzl T. E., Venkataramanan R. Role of bile and bile salts on cyclosporine absorption in dogs. Transplant Proc. 1987 Feb;19(1 Pt 2):1248–1249. [PMC free article] [PubMed] [Google Scholar]
- Frey F. J., Horber F. F., Frey B. M. Trough levels and concentration time curves of cyclosporine in patients undergoing renal transplantation. Clin Pharmacol Ther. 1988 Jan;43(1):55–62. doi: 10.1038/clpt.1988.11. [DOI] [PubMed] [Google Scholar]
- Grevel J., Nüesch E., Abisch E., Kutz K. Pharmacokinetics of oral cyclosporin A (Sandimmun) in healthy subjects. Eur J Clin Pharmacol. 1986;31(2):211–216. doi: 10.1007/BF00606661. [DOI] [PubMed] [Google Scholar]
- Henricsson S., Lindholm A., Johansson A. Identification of a sulfate conjugate of cyclosporin. Transplant Proc. 1989 Feb;21(1 Pt 1):837–838. [PubMed] [Google Scholar]
- Irschik E., Tilg H., Niederwieser D., Gastl G., Huber C., Margreiter R. Cyclosporin blood levels do correlate with clinical complications. Lancet. 1984 Sep 22;2(8404):692–693. doi: 10.1016/s0140-6736(84)91244-3. [DOI] [PubMed] [Google Scholar]
- Keogh A., Day R., Critchley L., Duggin G., Baron D. The effect of food and cholestyramine on the absorption of cyclosporine in cardiac transplant recipients. Transplant Proc. 1988 Feb;20(1):27–30. [PubMed] [Google Scholar]
- Klintmalm G., Säwe J., Ringdén O., von Bahr C., Magnusson A. Cyclosporine plasma levels in renal transplant patients. Association with renal toxicity and allograft rejection. Transplantation. 1985 Feb;39(2):132–137. doi: 10.1097/00007890-198502000-00005. [DOI] [PubMed] [Google Scholar]
- Lindholm A., Henricsson S., Lind M., Dahlqvist R. Intraindividual variability in the relative systemic availability of cyclosporin after oral dosing. Eur J Clin Pharmacol. 1988;34(5):461–464. doi: 10.1007/BF01046702. [DOI] [PubMed] [Google Scholar]
- Mehta M. U., Venkataramanan R., Burckart G. J., Ptachcinski R. J., Delamos B., Stachak S., Van Thiel D. H., Iwatsuki S., Starzl T. E. Effect of bile on cyclosporin absorption in liver transplant patients. Br J Clin Pharmacol. 1988 May;25(5):579–584. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T. M., Karmi S. A., Frantz S. C., Henriques H. F. Absorption profiles of renal allograft recipients receiving oral doses of cyclosporine: a pharmacokinetic study. Transplant Proc. 1988 Apr;20(2 Suppl 2):457–461. [PubMed] [Google Scholar]
- Ptachcinski R. J., Venkataramanan R., Rosenthal J. T., Burckart G. J., Taylor R. J., Hakala T. R. Cyclosporine kinetics in renal transplantation. Clin Pharmacol Ther. 1985 Sep;38(3):296–300. doi: 10.1038/clpt.1985.174. [DOI] [PubMed] [Google Scholar]
- Ptachcinski R. J., Venkataramanan R., Rosenthal J. T., Burckart G. J., Taylor R. J., Hakala T. R. The effect of food on cyclosporine absorption. Transplantation. 1985 Aug;40(2):174–176. doi: 10.1097/00007890-198508000-00013. [DOI] [PubMed] [Google Scholar]
- Quesniaux V., Tees R., Schreier M. H., Maurer G., van Regenmortel M. H. Potential of monoclonal antibodies to improve therapeutic monitoring of cyclosporine. Clin Chem. 1987 Jan;33(1):32–37. [PubMed] [Google Scholar]
- Quesniaux V., Tees R., Schreier M. H., Wenger R. M., Donatsch P., Van Regenmortel M. H. Monoclonal antibodies to ciclosporin. Prog Allergy. 1986;38:108–122. [PubMed] [Google Scholar]
- Reymond J. P., Steimer J. L., Niederberger W. On the dose dependency of cyclosporin A absorption and disposition in healthy volunteers. J Pharmacokinet Biopharm. 1988 Aug;16(4):331–353. doi: 10.1007/BF01062550. [DOI] [PubMed] [Google Scholar]
- Rogerson M. E., Marsden J. T., Reid K. E., Bewick M., Holt D. W. Cyclosporine blood concentrations in the management of renal transplant recipients. Transplantation. 1986 Feb;41(2):276–278. [PubMed] [Google Scholar]
- Shibata N., Minouchi T., Hayashi Y., Ono T., Shimakawa H. Quantitative determination of cyclosporin A in whole blood and plasma by high performance liquid chromatography. Res Commun Chem Pathol Pharmacol. 1987 Aug;57(2):261–271. [PubMed] [Google Scholar]
- Ueda C. T., Lemaire M., Gsell G., Misslin P., Nussbaumer K. Apparent dose-dependent oral absorption of cyclosporin A in rats. Biopharm Drug Dispos. 1984 Apr-Jun;5(2):141–151. doi: 10.1002/bdd.2510050207. [DOI] [PubMed] [Google Scholar]
- Ueda C. T., Lemaire M., Gsell G., Nussbaumer K. Intestinal lymphatic absorption of cyclosporin A following oral administration in an olive oil solution in rats. Biopharm Drug Dispos. 1983 Apr-Jun;4(2):113–124. doi: 10.1002/bdd.2510040203. [DOI] [PubMed] [Google Scholar]



