Abstract
Laboratory results provide necessary information for the management of ambulatory patients. To realize the benefits of an electronic health record (EHR) and coded laboratory data (e.g., decision support and improved data access and display), results from laboratories that are external to the health care enterprise need to be integrated with internal results. We describe the development and clinical impact of integrating external results into the EHR at Intermountain Health Care (IHC). During 2004, over 14,000 external laboratory results for 128 liver transplant patients were added to the EHR. The results were used to generate computerized alerts that assisted clinicians with managing laboratory tests in the ambulatory setting. The external results were sent from 85 different facilities and can now be viewed in the EHR integrated with IHC results. We encountered regulatory, logistic, economic, and data quality issues that should be of interest to others developing similar applications.
It is difficult to transition from paper to an electronic health record (EHR) when clinically important information charted on specialized paper records is only available in paper form. Clinicians want access to information and want decision support and other benefits associated with an electronic record. Laboratory test results are essential for managing ambulatory patients. A recent survey among clinicians in California identified electronic laboratory reporting in the ambulatory setting as a top priority.1 When clinicians monitor patients on different health plans or who reside in a wide geographic area, results may be reported from a variety of laboratories. Electronic reporting of laboratory results from external laboratories is feasible; however, EHRs typically only contain results from the enterprise's internal laboratory information system and from commercial laboratories used by the internal laboratory. Laboratories may e-mail or automatically fax printed reports, but this differs from sending structured, coded data that can be received into an EHR and used for decision support and various displays. In this case report, we describe logistic, financial, regulatory, and clinical issues that influenced our decision to manually enter external laboratory results into an EHR.
Case Description
The existing medical record system at Intermountain Health Care (IHC) includes paper records, paper flowcharts for special populations, and a longitudinal EHR that integrates information from multiple sources of electronic data.2 The EHR includes laboratory results from 21 IHC laboratories, including LDS Hospital in Salt Lake City, UT. Structured, coded laboratory data are stored in a central data repository and viewed using a Web-based application.
For 20 years, the Liver Transplant Program at LDS Hospital has used a paper flowchart to integrate IHC and external laboratory test results in chronological order so clinicians can view trends and drug level and dosage information side by side. Following surgery, transplant patients require lifelong monitoring of immunosuppression drug levels and blood chemistry results. The transplant patients use a variety of laboratories and reside throughout the Intermountain West. About 25% of the outpatient laboratory tests performed on over 300 liver transplant patients at LDS Hospital are reported by external laboratories. IHC and external laboratory reports are faxed or automatically printed to the transplant office and then transcribed to the paper flowchart by the nurses and medical assistants. The practice of transcribing laboratory results onto the paper flowchart is time-consuming and potentially error prone and does not meet the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) requirement that every clinical record entry be dated and its author identified.3
There are several advantages to storing coded laboratory data in an EHR. First, data in the EHR are accessible from inpatient and remote locations. Second, the EHR is permanent and access can be audited. Third, data can be arranged in different views. Fourth, electronic information can drive decision-support applications. The first two benefits can be realized by scanning faxed reports into the EHR; however, the second two benefits can only be realized if laboratory data are stored in a structured format using standardized codes.
There are several reasons that laboratory results external to IHC are not electronically transmitted to the IHC EHR. First, external laboratories have varying abilities and incentives to establish electronic interfaces with their customers. Most laboratory customers do not have an EHR to receive results. In 2001 to 2003, only 17% of physician offices and 29% of hospital outpatient departments had an EHR.4
Second, laboratory and care systems are rarely interoperable. Most laboratories use HL7 messages to send results, but laboratories use idiosyncratic codes to identify tests.5,6 Thus, the care system cannot fully understand the results they receive. They need to either adopt the producer's laboratory codes (which is impossible if they receive results from multiple sources), or map each result from a producer's code system to their internal code system.10 LOINC codes provide universal identifiers; however, these codes are not universally adopted.
Third, it is not logistically and economically feasible to establish interfaces with all laboratories performing tests for transplant patients. To store results in the correct patient record, every external laboratory would need to establish an interface with IHC and know the unique patient identifier for the IHC record system, or vice versa. Each unique interface developed at IHC is conservatively estimated to cost $15,000.
Transplant management information systems available from vendors did not meet our needs. Information was not stored back into the primary patient record in the EHR where it could be accessed by clinicians and decision-support applications. An application for entering external laboratory results into the EHR of transplant patients at Vanderbilt University Medical Center is briefly described in the literature.7 We were unable to find references that fully described a system for entering coded external laboratory data.
Method
We developed a system to meet the needs of the major stakeholders. Clinicians wanted to view all laboratory results in chronological order and be able to track new and overdue results. Medical assistants (MAs) and office staff wanted an intuitive user interface. Laboratory and health information personnel wanted to ensure that both IHC and external laboratory data in the EHR complied with national standards.3,8,9,10,11
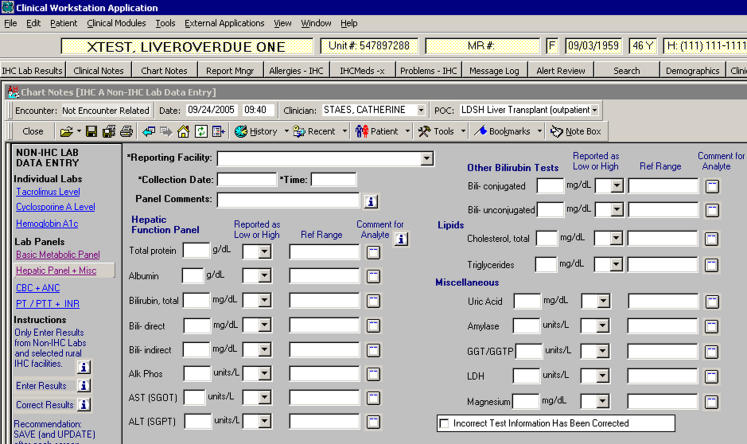
Five functional requirements influenced the system design. External results need to be entered, corrected, stored with IHC laboratory results, viewed, and appropriately interpreted. We developed data entry forms for tests routinely used by clinicians and reported by external laboratories (▶).12 The forms were designed for clerks with no formal training in laboratory science. For example, “info buttons,” labels with common acronyms, and data integrity functions were used. The data entry forms were designed to meet health record entry regulations and standards3,8,9 and laboratory reporting requirements specified by the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and the College of American Pathologists (CAP).10,11 CLIA and CAP require that laboratory reports include specific information, such as the name and location of the testing facility, test name and result, and reference ranges. The requirement pertaining to the testing facility was modified for reporting external laboratory results. We used the reporting facility rather than the testing facility if they differed. If more information was needed, clinicians would need to contact the reporting facility. The date and time that the record was created or modified and the name of the person making the entry were automatically determined by the system and saved with each record.
Figure 1.
Data entry form for the hepatic function panel and miscellaneous tests reported by external laboratories.
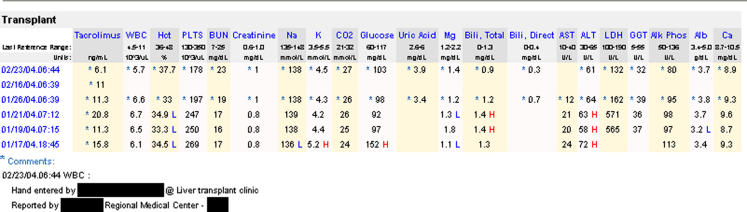
Clinicians could view external and IHC results in chronological order in the EHR (▶). Analytes were displayed in a “transplant” view defined in a configuration table. External laboratory results were marked with an asterisk and automatically included the following comments: “Reported by [reporting facility]” and “Hand entered by [person's name who entered the data] at [point of care]”.
Figure 2.
“Transplant” view of both IHC and external* laboratory results integrated in the patient's EHR. *The external laboratory results were collected January 26, 2004, February 16, 2004, and February 23, 2004.
In March 2004, staff were trained to use the data entry forms and the physicians and nurses were shown the “transplant” view of laboratory results. In June 2004, the LDS Hospital Transplant Program implemented alerts to identify liver transplant patients with new and overdue creatinine and immunosuppression drug levels. Computerized alerts were triggered when the interval between laboratory tests exceeded the time interval specified by the follow-up protocol. Subsequently, we assessed utilization, clinical relevance, and the quality of the external results in the EHR.
Example
Between June 1 and December 31, 2004, 14,082 analytes for 594 basic chemistry tests, 576 complete blood counts, 569 basic metabolic panels, 344 tacrolimus levels, 48 coagulation panels, 31 cyclosporin A levels, and 9 hemoglobin A1c tests were saved to the EHR for 128 liver transplant patients. The reporting laboratories included mobile, small and large laboratories associated with hospitals, clinics, home health agencies, physician's offices, and national referral laboratories. Results were reported by 85 external laboratories, with no more than 7% of the results reported by any one laboratory. During the next six months (January to July 2005), 19 additional external laboratories reported results and 18 previously reporting laboratories were no longer used.
External results in the EHR were essential for tracking compliance with follow-up laboratory testing. Both external and IHC laboratory results were processed to identify the absence of results. Among 298 patients with laboratory results that created new laboratory alerts between June 15 and December 31, 2004, 56 (19%) patients had both external and IHC results, and 66 (22%) patients had only external results. During this time period, the clinicians received alerts for patients overdue for an immunosuppression drug level (n = 152) and a creatinine test (n = 141).
According to a survey conducted on April 12, 2005 (86% response rate), the 12 physicians and nurses reported that they “always” or “usually” (92%) used the EHR when they did not have access to the paper flowchart. This situation occurred “daily” (33%), “one to five times per week” (42%), or “one to five times per month” (25%). The clinicians requested continued entry of external laboratory results.
External laboratory records in the EHR were found to be more complete than the paper records and contained few errors. In March 2005, we compared the date and value of creatinine, cyclosporin A, and tacrolimus levels reported on the laboratory reports with information entered into the EHR and onto the paper flowcharts. Among 163 results “charted” during a three-week period, one result in the EHR had an erroneous specimen collection date and three results on a paper flowchart had an erroneous specimen collection date or value. We queried 14,082 external results entered between June 1 and December 31, 2004, and identified four records with nonsensical collection dates and eight records that had already been recognized and corrected. We assessed the minimum, maximum, and mean values charted for each analyte and compared the results with applicable reference ranges. We identified four abnormal values that were clearly transcription errors and 19 absolute neutrophil count results with incorrect units of measure. Reference ranges were charted so clinicians could interpret the results. We found that 87% of the results had information recorded in the reference range field, a substantial improvement over the flowchart where none of this information was charted. We identified four external laboratories that routinely sent specimens to an IHC laboratory for drug level testing and then reported the results with their other reports. Thus, these results were sometimes duplicated in the EHR, which is problematic when viewing trends.
Discussion
We met our goal to integrate external and IHC laboratory results in the EHR so results could be used for clinical decision making. After nine months of experience, external results continue to be entered, viewed, and used for alerts. While we identified some transcription errors and problems with specific analytes, this project demonstrated the feasibility and benefit of manual data entry.
While establishing electronic interfaces with external laboratories is technically feasible and preferable,5,6 it is not logistically or economically feasible in many cases. The external laboratories were heterogeneous, and no one laboratory reported more than 7% of the results. Given that HL7 version 2 does not result in interoperability and there are no national standards for naming laboratory tests, receiving all laboratory data electronically would have required the creation of over 85 unique interfaces. Using a conservative estimate of $15,000 per interface, the project would initially cost IHC over $1.25 million. Over time, new interfaces would need to be added and existing interfaces would be unnecessary as patients move, die, or use other laboratories. Moreover, most of the external facilities do not have the resources or desire to establish an electronic interface with IHC.
Even if costs were reduced and interoperability were enhanced with new standards,1,5,6 systems would need to be established to ensure that results are stored in the correct record when test orders originate from a system external to IHC. The major new initiative (EHR-Laboratory Interoperability and Connectivity Standard) only addresses transmission back to the ordering entity.1 In the meantime, these standards are still under development and most laboratories mail or fax laboratory reports. Therefore, there continues to be a need to hand-enter results while clinicians need integrated information in the ambulatory EHR.
The primary author (CJS) was supported with funding from a Medical Informatics Training Grant from the National Library of Medicine and directly from Intermountain Health Care.
This project was approved by Institutional Review Boards from both the University of Utah and Intermountain Health Care.
References
- 1.EHR-Lab Interoperability and Connectivity Standards (ELINCS). Developing a national lab data standard for EHRs. [online] 2005 [cited 2005 May 25]. Available from: http://www.chcf.org/topics/chronicdisease/index.cfm?itemID=108868#.
- 2.Clayton PD, Narus SP, Huff SM, Pryor TA, Haug PJ, Larkin T, et al. Building a comprehensive clinical information system from components. The approach at Intermountain Health Care. Methods Inf Med. 2003;42:1–7. [PubMed] [Google Scholar]
- 3.Practice brief. Authentication of health record entries (updated). American Healthcare Information Management Association. J AHIMA. 2000;71:68A–G. [PubMed] [Google Scholar]
- 4.Burt C, Hing E. Use of computerized clinical support systems in medical settings: United States, 2001–03. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics, 2005. Advance Data no. 353. Available from: http://www.cdc.gov/nchs/data/ad/ad353.pdf. [PubMed]
- 5.HL7 Reference Information Model version 2.02 [online] 2003 [cited 2004 Aug 8]. Available from: http://www.hl7.org/.
- 6.Regenstrief Institute, Inc. Logical Observation Identifiers Names and Codes (LOINC®) [online] 2004 [cited 2005 Apr 16]. Available from: http://www.regenstrief.org/loinc.
- 7.Sarkar MS, Giuse SD, Ing D, Shultz E, Miller RA. Supporting longitudinal care for transplant patients with an external laboratory data entry application. Proc AMIA Annu Fall Symp. 2001:, Available from: http://www.amia.org/pubs/proceedings/symposia/2001/D010001376.pdf.
- 8.Comprehensive Accreditation Manual for Hospitals: The Official Handbook, 2005. Oakbrook Terrace, IL: Joint Commission on Accreditation of Healthcare Organizations, 2005.
- 9.Dougherty M. Maintaining a legally sound health record (AHIMA Practice Brief). J AHIMA. 2002;73:64A–G. [PubMed] [Google Scholar]
- 10.Laboratory Requirements. 42 CFR 493.1109 Standard; Test report. 2003 [cited 2005 February 15]; Available from: http://www.access.gpo.gov/nara/cfr/waisidx_00/42cfr493_00.html.
- 11.Commission on Laboratory Accreditation. Laboratory accreditation program. Laboratory general checklist. 2004 12/28/2004 [cited 2005 Feb 4]. Available from: http://www.cap.org/apps/docs/laboratory_accreditation/checklists/laboratory_general_december2004.pdf.
- 12.Staes C. Development of an information system for the management of liver transplant patients [dissertation]. Salt Lake City: University of Utah, 2005, (in progress).