Abstract
A coding single-nucleotide polymorphism (cSNP), K172N, in hTAS2R16, a gene encoding a taste receptor for bitter β-glucopyranosides, shows significant association with alcohol dependence (P=.00018). This gene is located on chromosome 7q in a region reported elsewhere to exhibit linkage with alcohol dependence. The SNP is located in the putative ligand-binding domain and is associated with an increased sensitivity to many bitter β-glucopyranosides in the presence of the N172 allele. Individuals with the ancestral allele K172 are at increased risk of alcohol dependence, regardless of ethnicity. However, this risk allele is uncommon in European Americans (minor-allele frequency [MAF] 0.6%), whereas 45% of African Americans carry the allele (MAF 26%), which makes it a much more significant risk factor in the African American population.
Alcohol dependence (MIM 103780) is one of the most common and costly health problems in the United States (Centers for Disease Control and Prevention 2004). It is a complex disease, with both genetic and environmental contributions to the risk. Family, adoption, and twin studies provide convergent evidence of hereditary factors in alcoholism (Heath et al. 1997). Heritable influences account for ∼40%–60% of the total variance in risk (Pickens et al. 1991; Kendler et al. 1994). The Collaborative Study of the Genetics of Alcoholism (COGA) was established to identify genes that modify susceptibility to alcoholism and related phenotypes. Genomewide linkage analyses using COGA pedigrees have provided consistent evidence of an alcoholism-susceptibility locus on the long arm of chromosome 7 in both the initial data set (Reich et al. 1998) and the replication data set (Foroud et al. 2000). Our recent studies have also shown linkage of an overlapping region of chromosome 7q with major depressive disorder (MIM 608516), composite phenotypes of alcohol dependence and/or depression, and electrophysiological measures derived from event-related oscillations (Nurnberger et al. 2001; Jones et al. 2004; Wang et al. 2004). Evidence of genetic linkage to alcohol dependence has also been reported in two Native American populations (Long et al. 1998; Ehlers et al. 2004) and in extended families from the Framingham Heart Study population (Ma et al. 2003), although none of these studies showed linkage to chromosome 7q.
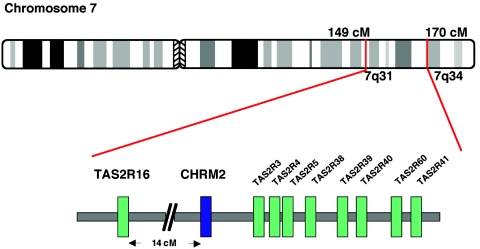
Elsewhere, we have reported evidence of association between individual SNPs and specific haplotypes within the gene encoding the acetylcholine muscarinic receptor 2 (CHRM2 [MIM 118493]) and alcohol dependence as well as major depressive syndrome (Wang et al. 2004). Since this gene lies near the edge of the linkage peak, we suspected that additional alcoholism-susceptibility loci exist in this region of chromosome 7. A search of the public databases revealed a cluster of bitter-taste receptors (TAS2Rs) in this region, which are potential candidate genes. The TAS2R genes, with a size range of 876–1,014 bp, have intronless coding regions, code for G protein–coupled receptors, and have recently been identified in mice and in humans (Adler et al. 2000; Matsunami et al. 2000). A number of coding SNPs (cSNPs) have been identified in human bitter-taste–receptor genes (Ueda et al. 2001; Kim et al. 2003, 2005; Soranzo et al. 2005). Among these, three cSNPs in the hTAS2R38 gene (MIM 607751 and MIM 171200) and one cSNP in the hTAS2R16 gene (MIM 604867) have been shown to alter receptor functions or taste sensitivity to bitter compounds, which suggests that genetic variation of these TAS2Rs may correlate with susceptibility to diet-related disease (Tepper 1998; Kim et al. 2003; Wooding et al. 2004; Bufe et al. 2005; Soranzo et al. 2005). Furthermore, variation in the hTAS2R38 gene has been associated with drinking behavior but not alcohol dependence (Duffy et al. 2004a, 2004b). Whereas the hTAS2R38 and other members of the cluster are located telomeric to the CHRM2 gene (fig. 1), the hTAS2R16 gene is located between the CHRM2 gene and our linkage peak. In this study, we genotyped the entire COGA linkage sample with four SNPs within and flanking the hTAS2R16 gene, including two nonsynonymous cSNPs (K172N and R222H), and examined the association between these variations and alcohol dependence.
Figure 1.
Location of the cluster of nine TAS2R genes on chromosome 7q (not drawn to scale).
Material and Methods
Study Subjects and Assessment
Linkage sample.—Alcohol-dependent probands, defined by DSM-IIIR alcohol dependence (American Psychiatric Association 1987) and Feighner-criteria for definite alcoholism (Feighner et al. 1972), were systematically recruited from alcohol-treatment units, and their biological relatives were invited to participate in the study. All subjects were assessed using the Semi-Structured Assessment for the Genetics of Alcoholism (Bucholz et al. 1994; Hesselbrock et al. 1999), a semi-structured interview designed as a polydiagnostic instrument that generates Feighner, DSM-IIIR, DSM-IV (American Psychiatric Association 1994), and ICD-10 (World Health Organization 1993) diagnoses of alcohol dependence. These diagnoses are essentially nested, with DSM-IIIR and Feighner definite alcoholism defining the broadest diagnosis, and ICD-10, the narrowest definition of dependence (Culverhouse et al. 2005). Informed consent was obtained from all subjects. A total of 262 families—including 2,310 individuals, with an average of 4.6 alcohol-dependent individuals per pedigree—were selected for genetic-linkage studies. Among these pedigrees, 298 individuals from 35 pedigrees are African American, and 8 pedigrees are of mixed ancestry (by self-report).
Additional trios.—The COGA sample contains additional pedigrees with cell lines that were not informative for linkage and had therefore not been selected for the linkage sample. From these, we identified 85 trios consisting of a DSM-IV–defined alcohol-dependent individual and two parents. This sample of “additional trios,” including five African American trios, was typed for SNP rs846664.
Identity-by-Descent (IBD) Sharing
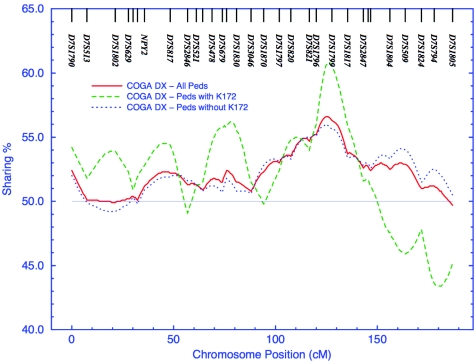
Nonparametric multipoint linkage analysis of independent (n-1) affected sibling pairs was conducted using ASPEX, which allows large sibships to be included in analyses. Linkage analyses were performed using the SIBPHASE option, which infers allele sharing if there is ambiguity between identity by state and IBD, by use of marker frequencies in the sample. To avoid biases due to ethnic stratification, maximum-likelihood allele-frequency estimates were obtained, from the USERM13 subroutine of MENDEL (Boehnke 1991), separately for African American and European American pedigrees. Maximum-likelihood estimates of sharing are displayed in figure 2.
Figure 2.
Affected-sibling-pair sharing on chromosome 7. Sharing computed, by ASPEX sib_phase, using all parents, with large pedigrees down-weighted to n-1. The solid line represents all pedigrees; the dotted line represents pedigrees in which no individual has a copy of the rare polymorphism; the dashed line represents pedigrees in which at least one individual has a copy of the rare polymorphism.
Association Analysis
Transmit (Clayton 1999), an extension of the transmission/disequilibrium test (Spielman and Ewens 1996) used to test for association in extended pedigrees to allow for missing parental genotypes, was used to test each SNP individually for evidence of linkage and association. The three closely correlated alcohol-dependence phenotypes—DSM-IIIR and Feighner definite alcoholism, DSM-IV alcohol dependence, and ICD-10 alcohol dependence—were tested to examine the consistency of results. For the additional trios, association was first tested, using Transmit, in this sample alone and was then computed again when combined with the linkage sample.
SNP Assays
The dbSNP database was used to identify SNPs within and flanking the hTAS2R16 gene. Both pyrosequencing (Biotage Pyrosequencing) and mass spectrometry (Sequenom) methods were used for SNP genotyping. For pyrosequencing, PCR primers were selected using the MacVector 6.5.3 program (Accelrys) to yield 200–500-bp genomic fragments containing the SNP. Standard procedures were followed to generate PCR products. Sequencing primers were designed using the Pyrosequencing Primer Design program. For mass spectrometry, PCR primers, termination mixes, and multiplexing capabilities were determined with Sequenom Spectro Designer software v2.00.17. Standard PCR procedures were used to amplify PCR products. All unincorporated nucleotides were deactivated with shrimp alkaline phosphatase. A primer-extension reaction was then performed with the mass-extension primer and the appropriate termination mix. The primer-extension products were then cleaned with resin and were spotted onto a silicon SpectroChip. The chip was scanned by mass spectrometry (Bruker), and the resulting genotype spectra were analyzed with the Sequenom SpectroTYPER software.
Sequence Analysis to Identify Additional Variants
The entire coding region of hTAS2R16 was sequenced in both directions in DNA from 14 people, including one European American and six African Americans homozygous for the minor allele of rs846664 and seven African Americans heterozygous for the same SNP. Publicly available sequence databases were used to select PCR primers, to amplify the coding exon plus at least 60 bp of flanking intronic sequence. The PCR product was purified using a QIAquick PCR purification kit (Qiagen) to remove excess primers. Purified PCR product was sequenced using the BigDye Terminator Cycle Sequencing method and then was electrophoresed on an ABI3100 automated DNA sequencer (Applied Biosystems [ABI]). Electropherograms were analyzed using ABI DNA Sequencing Analysis Software, version 3.4.
Heterologous Expression
Generation of the hTAS2R16 haplotypes and functional analysis in HEK293 cells were performed as described elsewhere (Bufe et al. 2002; Soranzo et al. 2005).
Results
The entire COGA linkage sample was genotyped with four SNPs, including two nonsynonymous cSNPs, K172N (rs846664) and R222H (rs860170). Since the three cSNPs showed dramatic differences in allele frequency between African Americans and European Americans, we stratified samples by race for all analyses (table 1A). Three SNPs were in Hardy-Weinberg equilibrium (HWE) in the founders of the stratified subsets. SNP rs846664 has a very low minor-allele frequency (MAF) in European Americans and is not in HWE in this sample, but it is in HWE in the African American samples, in which the MAF is much higher. We used Transmit to determine the pairwise disequilibrium between the SNPs and observed high levels of linkage disequilibrium (LD) (D′⩾0.89). Transmit was also used to test each SNP individually for evidence of association between the SNPs and the alcohol-dependence phenotypes. The nonsynonymous cSNP rs846664 showed significant association with all three correlated alcohol-dependence diagnoses in the COGA linkage sample (table 1B). This association appears to be driven by the African American subset (P=.004 for DSM-IV dependence); however, the non–African Americans clearly contribute to the significance of the P value when the polymorphism is present, because the overall significance is substantially greater in the combined sample (P=.0008 for DSM-IV dependence), indicating that the rs846664 polymorphism is also overtransmitted, when it occurs, in non–African American populations (table 1B). In the linkage sample, a trend of association was also observed between the synonymous cSNP rs1204014 and DSM-IV alcohol dependence. The significance of this association increased (P=.003) when the test for association was restricted to the subset of African American families. Neither the noncoding SNP rs978739 nor the nonsynonymous cSNP rs860170 showed any association with alcohol dependence in our sample. Haplotype analyses using the two nonsynonymous cSNPs (rs846664 and rs860170) and the two significantly associated SNPs (rs846664 and rs1204014) were less significant than the single SNP association results for rs846664 (data not shown).
Table 1.
Association of Alcohol-Dependence Diagnoses with SNPs in hTAS2R16 in the COGA Linkage Sample
| A. MAFs of Each SNP in Different Sample Sets | ||||
| MAFa for SNP |
||||
| Sample (No. of Families) | rs978739(noncoding) | rs846664(K172N) | rs860170(R222H) | rs1204014(T282T) |
| All families (262) | .35 | .04 | .30 | .08 |
| African American families (35) | .34 | .26 | .10 | .27 |
| European American families (219) | .35 | .006 | .32 | .05 |
| B. Association of Alcohol-Dependence Diagnoses with SNPs in hTAS2R16 in the COGA Linkage Sample | ||||
|
P Valuec for SNP |
||||
| Sample and Alcohol-Dependence Diagnosisb | rs978739(noncoding) | rs846664(K172N) | rs860170(R222H) | rs1204014(T282T) |
| All families: | ||||
| COGA (N=1,065) | .277 | .008 | .512 | .256 |
| DSM-IV (N=909) | .114 | .0008 | .307 | .051 |
| ICD10 (N=683) | .369 | .006 | .336 | .186 |
| African American: | ||||
| COGA (N=128) | .695 | .024 | .547 | .028 |
| DSM-IV (N=112) | .859 | .004 | .913 | .003 |
| ICD10 (N=87) | .945 | .066 | .843 | .116 |
| European American: | ||||
| COGA (N=907) | .193 | .593 | .378 | .608 |
| DSM-IV (N=768) | .061 | .626 | .252 | .826 |
| ICD10 (N=574) | .297 | .890 | .260 | .840 |
Allele frequencies were calculated from founders only.
N = total number of individuals with diagnosis.
P values were computed using Transmit. Significant P values are in bold italics.
To further explore the role of SNP rs846664, we stratified the linkage sample into those families containing the minor allele K172 and those without and performed affected-sibling-pair linkage analysis with ASPEX. The families, including 62 nuclear pedigrees, with the minor allele exhibited IBD sharing of 61.0% at the linkage peak (D7S1799) on chromosome 7 for DSM-IIIR and Feighner definite alcoholism, whereas families, including 344 nuclear pedigrees, without the K172 allele exhibited IBD sharing of 55.7% (overall sharing is 56.5%) (fig. 2). Thus, it appears that, although only 15% of the nuclear families have one or more individuals carrying the minor allele, those families contribute disproportionately to the linkage signal on chromosome 7. The procedure of comparing IBD sharing in pedigrees with and without a putative risk allele is conservative and may, in fact, underestimate the effect of the allele (Li et al. 2004).
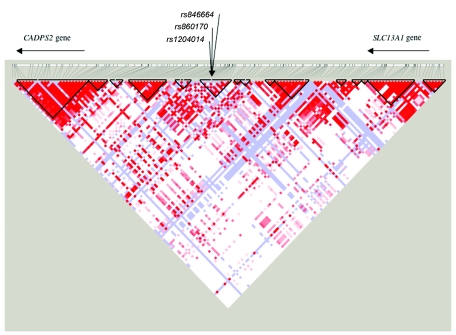
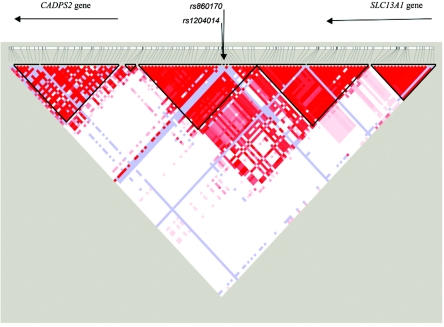
To extend our results with rs846664, we genotyped this SNP in an independent sample: 85 trios (consisting of a DSM-IV alcohol-dependent individual and two parents) (table 2). With use of Transmit, the independent trios showed an overtransmission of the K172 allele, with a trend of association with DSM-IV alcohol dependence. When the data from the 85 trios were combined with that of the linkage sample, we observed a P value of .00018 for DSM-IV dependence and substantial overtransmission of the K172 allele (79 observed/62 expected transmission). Strong association was also detected with the correlated alcohol-dependence diagnoses—DSM-IIIR and Feighner definite alcoholism (P=.002) and ICD-10 dependence (P=.002). Sequencing of the coding region of the hTAS2R16 gene in individuals homozygous and heterozygous for the minor allele (K172) confirmed that there were two nonsynonymous coding changes in the gene—the lysine→asparagine mutation at codon 172 (rs846664) and the arginine→histidine mutation at codon 222 (rs860170)—and a synonymous cSNP at codon 282 (rs1204014) (fig. 3). No additional SNPs were observed. Given the LD pattern in both European Americans and Africans derived from the International HapMap Project and the fact that the neighboring genes (CADPS2 and SLC13A1) are each >100 kb from the hTAS2R16 gene (figs. 4 and 5), it is very likely that the alcoholism-susceptibility locus detected in the present study is within hTAS2R16.
Table 2.
Association of DSM-IV Alcohol Dependence with SNP rs846664
| Sample | No. of NuclearPedigrees/Affected Offspring | No. of Pedigrees with Heterozygous Parents | No. of Observed/Expected Transmissions | P Valuea |
| COGA linkage sample | 383/758 | 23 | 76/61 | .0008 |
| Additional trios | 85/85 | 3 | 3/1.5 | .083 |
| Combined sample: | 468/843 | 26 | 79/62 | .00018 |
| African American subset | 53/96 | 17 | 59/47.5 | .0011 |
| European American subset | 398/720 | 5 | 6/6.8 | .649 |
P values were computed using Transmit.
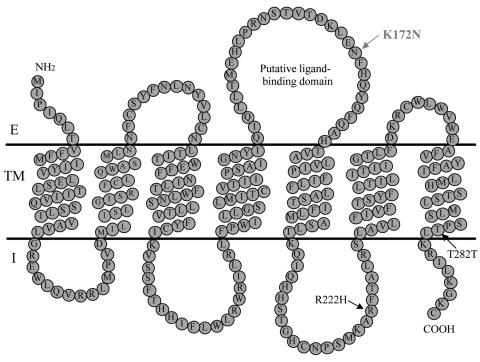
Figure 3.
Predicted topology of hTAS2R16. E = extracellular domain; TM = transmembrane domain; I = intracellular domain.
Figure 4.
Pairwise LD between markers flanking three SNPs in TAS2R16 (500 kb) from Yoruba in population from Ibadan, Nigeria.
Figure 5.
Pairwise LD between markers flanking two SNPs in TAS2R16 (500 kb) from CEPH (Utah residents with ancestry from northern and western Europe) population.
The K172N substitution rs846664 is located in the extracellular loop 2 between transmembrane domains 4 and 5 (fig. 3). In G protein–coupled receptors, including the TAS2Rs, this domain has been associated with ligand binding (Adler et al. 2000; Pronin et al. 2004). Moreover, experimental evidence has demonstrated that the extracellular loop 2 is involved in the activation of the bitter-taste receptor hTAS2R43 by its agonist 6–nitrosaccharin (Pronin et al. 2004), which suggests that the K172N substitution in the extracellular loop 2 may alter receptor signaling/taste perception. To directly test whether K172N and/or R222H influence hTAS2R16 function, we expressed cDNAs coding for the three hTAS2R16 haplotypes N172 + R222, N172 + H222, and K172 + H222 in HEK293T cells, and we performed functional assays with four bitter-taste agonists as described elsewhere (Bufe et al. 2002; Soranzo et al. 2005). We did not analyze the fourth predicted haplotype (K172 + R222) because it was not observed in the entire COGA data set. The concentrations of half-maximal responses (EC50) for the N172 + R222 and N172 + H222 constructs were not significantly different for any of the four structurally divergent β-glucopyranosides. In contrast, the K172 + H222 construct exhibited a higher EC50 for three of the four compounds tested (table 3). These results show that the K172N polymorphism results in a functional change to the receptor, whereas no functional effect of the R222H polymorphism was observed.
Table 3.
Average EC50 Values of hTAS2R16 Variants for Four Bitter-Taste Agonists[Note]
| EC50 Values (mM) for Haplotype |
P Value for t Test |
|||||
| Agonist | N172 + R222 | N172 + H222 | K172 + H222 | NR/KH | NH/KH | NR/NH |
| 8-Hydroxyquinoline-β-d-glucoside | .6±.3 | .7±.4 | 1.6±.8 | .002 | .012 | .302 |
| Helicin | 1.7±.9 | 1.8±.8 | 2.9±.9 | .017 | .025 | .796 |
| Phenyl-β-d-glucoside | 1.0±.3 | .9±.2 | 1.5±.6 | .024 | .003 | .249 |
| n-Hexyl-β-d-glucoside | 1.6±.9 | 1.6±.8 | 2.0±1.0 | .227 | .294 | .884 |
Note.— Significant P values are in bold italics.
Discussion
Elsewhere, we examined a candidate gene, CHRM2, near the linkage peak for alcohol dependence on chromosome 7 and reported evidence of association between genetic variation in this gene and alcohol dependence and related phenotypes (Jones et al. 2004; Wang et al. 2004). However, this gene is at the edge of our linkage peak, and several analyses suggest that this association cannot account totally for the observed linkage. Since there are probably many genetic factors influencing the development of alcoholism, we pursued other candidate genes in this region, specifically the hTAS2r16 bitter-taste–receptor gene.
Our SNP analysis with Transmit detected a significant association between the nonsynonymous cSNP K172N (rs846664) in hTAS2R16 and all three nested alcohol-dependence diagnoses in the COGA linkage sample. To extend this result, we genotyped this variant in an independent sample of 85 trios. The association in the trios showed a trend only in the same direction as the original sample, most likely because of the small sample size. When the trios were combined with the linkage sample, the evidence of association increased. After a Bonferroni correction for 12 tests in the overall linkage sample (four SNPs and three alcoholism diagnoses), the DSM-IV diagnosis remained significant. Although the four SNPs are essentially uncorrelated because of differences in allele frequencies, the overall correction is overly conservative, since the alcoholism diagnoses are highly correlated. The analyses of allele-frequency differences in subpopulations were exploratory and would not be significant after a Bonferroni correction.
The LD structure in this region in both European Americans and Africans indicates that the TAS2R16 gene is the only gene in the LD block containing the SNP rs846664. Furthermore, the neighboring genes are >100 kb away from hTAS2R16. These observations strongly support the hypothesis that the alcoholism-susceptibility locus detected in this study is within the hTAS2R16 gene. Since no novel cSNPs were identified from our sequencing analysis and no association was observed between cSNP R222H and alcohol dependence, both our genetic analysis and the heterologous expression studies suggests that the K172 allele is the functional variant.
The K172 allele is uncommon in the European Americans in our sample (with an MAF of 0.6%), but 45% of African Americans in our sample carry this allele (MAF 26%). To assess the distribution of the K172 allele across multiple populations, we typed this SNP in the Human Genome Diversity Project–CEPH Human Genome Diversity Cell Line Panel, which includes 1,057 individuals and represents 52 different populations (Cann et al. 2002). The MAF for rs846664 had a range of 10%–44% in African populations, but it was not detected or was present at very low frequency in non-African populations (table 4). These frequency data and our own findings that the K172 allele is associated with alcoholism in African American pedigrees (tables 1 and 2) suggest that this SNP is likely to be a much more significant risk factor for alcoholism among populations of African origin.
Table 4.
Allele Frequency Distribution (%) of cSNP rs846664 in Different Ethnic Populations
| Population (No. of Subjects) | Allele K172 | Allele N172 |
| Yoruba (24) | 44 | 56 |
| Bantu S.E. (8) | 31 | 69 |
| Biaka Pygmy (36) | 21 | 79 |
| Mandenka (24) | 21 | 79 |
| Mbuti Pygmy (15) | 20 | 80 |
| Bantu N.E. (12) | 17 | 83 |
| San (7) | 14 | 86 |
| Mozabite (30) | 10 | 90 |
| Palestinian (50) | 6 | 94 |
| Maya (25) | 4 | 96 |
| Bedouin (47) | 2 | 98 |
| Brahui (24) | 2 | 98 |
| Adygei (17) | 0 | 100 |
| Balochi (26), Makrani (26), Sindhi (24), Burusho (25), Hazara (22), Kalash (25), Pathan (25) | 0 | 100 |
| Basque (30), French (24) | 0 | 100 |
| Bergamo (27), Sardinian (14), Tuscan (8) | 0 | 100 |
| Cambodian (11) | 0 | 100 |
| Colombia (13) | 0 | 100 |
| Dai (10), Lahu (10), Naxi (10) | 0 | 100 |
| Daur (10) | 0 | 100 |
| Druze (47) | 0 | 100 |
| Han (45) | 0 | 100 |
| Hezhen (10), Oroqen (10), Tu (10) | 0 | 100 |
| Japanese (31) | 0 | 100 |
| Karitiana (24) | 0 | 100 |
| Miaozu (10), Uygur (10), Xibo (10) | 0 | 100 |
| Mongola (10) | 0 | 100 |
| Melanesian (22) | 0 | 100 |
| Orcadian (16) | 0 | 100 |
| Papuan (17) | 0 | 100 |
| Pima (25) | 0 | 100 |
| Russian (25) | 0 | 100 |
| She (10), Tujia (10), Yizu (10) | 0 | 100 |
| Surui (21) | 0 | 100 |
| Yakut (25) | 0 | 100 |
Comparative sequence analysis of several primate species indicates that the ancestral allele at residue 172 of the hTAS2R16 gene is, in fact, the K172 allele, which is the minor allele in all human populations examined to date (table 4) (Soranzo et al. 2005). The presence of the minor human allele (K172) in all primate species that have been sequenced suggests that the N172 allele must have undergone massive positive selection during human evolution. The N172 allele is associated with an increased sensitivity to bitter β-glucopyranosides such as salicin, arbutin, and cyanogenic glycosides (Soranzo et al. 2005). Our functional studies confirm the association between the N172 allele and increased sensitivity to bitter β-glucopyranosides (table 3). The increased sensitivity to bitter compounds in the diet may have driven the positive selection of this allele (Soranzo et al. 2005). These results suggest that the bitter taste of some alcoholic drinks may influence drinking habits and that lack of this bitter-taste variant is a susceptibility factor for alcoholism. However, direct measurements of the perception of bitter taste in subjects homozygous for the K172 or N172 alleles with different bitter-taste agonists and a detailed analysis of their drinking habits are needed to further examine the influence of these alleles on taste perception.
In summary, our genetic and functional data demonstrate that the K172 allele of the polymorphism rs846664 within the putative ligand-binding domain of the hTAS2R16 receptor reduces sensitivity of the receptor to bitter-taste stimuli and may thereby influence susceptibility to alcohol dependence.
Acknowledgments
The COGA (principal investigator: H. Begleiter; coprincipal investigators: L. Bierut, H. Edenberg, V. Hesselbrock, and B. Porjesz) includes nine different centers where data collection, analysis, and storage take place. The nine sites and principal investigators and coinvestigators are: University of Connecticut (V. Hesselbrock); Indiana University (H. Edenberg, J. Nurnberger Jr., P. M. Conneally, and T. Foroud); University of Iowa (S. Kuperman and R. Crowe); State University of New York Health Science Center at Brooklyn (B. Porjesz and H. Begleiter); Washington University (L. Bierut, A. Goate, and J. Rice); University of California at San Diego (M. Schuckit); Howard University (R. Taylor); Rutgers University (J. Tischfield); and Southwest Foundation (L. Almasy). Zhaoxia Ren serves as the National Institute on Alcohol Abuse and Alcoholism (NIAAA) staff collaborator. This national collaborative study is supported by National Institutes of Health grant U10AA08401 from the NIAAA. W. Meyerhof is the recipient of a grant from the German Science Foundation. In memory of Theodore Reich, coprincipal investigator of COGA since its inception and one of the founders of modern psychiatric genetics, we acknowledge his immeasurable and fundamental scientific contributions to COGA and the field.
Web Resources
The URLs for data presented herein are as follows:
- ASPEX, http://aspex.sourceforge.net/ (for affected sib-pair exclusion mapping)
- Biotage Pyrosequencing, http://www.pyrosequencing.com/
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/
- International HapMap Project, http://www.hapmap.org/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for alcoholism, depressive disorder, CHRM2, hTAS2R38, and hTAS2R16)
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS (2000) A novel family of mammalian taste receptors. Cell 100:693–702 10.1016/S0092-8674(00)80705-9 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1987) Diagnostic and statistical manual of mental disorders, 3rd ed (revised). American Psychiatric Press, Washington, DC, pp 166–175 [Google Scholar]
- ——— (1994) Diagnostic and statistical manual of mental disorders, 4th ed. American Psychiatric Press, Washington, DC, pp 194–196 [Google Scholar]
- Boehnke M (1991) Allele frequency estimation from data on relatives. Am J Hum Genet 48:22–25 [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI Jr, Reich T, Schmidt I, Schuckit MA (1994) A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 55:149–158 [DOI] [PubMed] [Google Scholar]
- Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W (2005) The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol 15:322–327 10.1016/j.cub.2005.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufe B, Hofmann T, Krautwurst D, Raguse JD, Meyerhof W (2002) The human TAS2R16 receptor mediates bitter taste in response to β-glucopyranosides. Nat Genet 32:397–401 10.1038/ng1014 [DOI] [PubMed] [Google Scholar]
- Cann HM, de Toma C, Cazes L, Legrand MF, Morel V, Piouffre L, Bodmer J, et al (2002) A human genome diversity cell line panel. Science 296:261–262 10.1126/science.296.5566.261b [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2004) Alcohol-attributable deaths and years of potential life lost—United States, 2001. MMWR Morb Mortal Wkly Rep 53:866–870 [PubMed] [Google Scholar]
- Clayton D (1999) A generalization of the transmission/disequilibrium test for uncertain-haplotype transmission. Am J Hum Genet 65:1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culverhouse R, Bucholz KK, Crowe RR, Hesselbrock V, Nurnberger JI Jr, Porjesz B, Schuckit MA, Reich T, Bierut LJ (2005) Long-term stability of alcohol and other substance dependence diagnoses and habitual smoking: an evaluation after 5 years. Arch Gen Psychiatry 62:753–760 10.1001/archpsyc.62.7.753 [DOI] [PubMed] [Google Scholar]
- Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC, Pakstis AJ, Reed DR, Snyder DJ, Bartoshuk LM (2004a) Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res 28:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy VB, Peterson JM, Bartoshuk LM (2004b) Associations between taste genetics, oral sensation and alcohol intake. Physiol Behav 82:435–445 10.1016/j.physbeh.2004.04.060 [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Wall TL, Phillips E, Feiler H, Wilhelmsen KC (2004) Genomic screen for loci associated with alcohol dependence in Mission Indians. Am J Med Genet B Neuropsychiatr Genet 129:110–115 10.1002/ajmg.b.30057 [DOI] [PubMed] [Google Scholar]
- Feighner JP, Robins E, Guze SB, Woodruff RA Jr, Winokur G, Munoz R (1972) Diagnostic criteria for use in psychiatric research. Arch Gen Psychiat 26:57–63 [DOI] [PubMed] [Google Scholar]
- Foroud T, Edenberg HJ, Goate A, Rice J, Flury L, Koller DL, Bierut LJ, Conneally PM, Nurnberger JI, Bucholz KK, Li TK, Hesselbrock V, Crowe R, Schuckit M, Porjesz B, Begleiter H, Reich T (2000) Alcoholism susceptibility loci: confirmation studies in a replicate sample and further mapping. Alcohol Clin Exp Res 24:933–945 10.1097/00000374-200007000-00001 [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG (1997) Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med 27:1381–1396 10.1017/S0033291797005643 [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V (1999) A validity study of the SSAGA—a comparison with the SCAN. Addiction 94:1361–1370 10.1046/j.1360-0443.1999.94913618.x [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut LJ, Goate A, Wang JC, Hinrichs, et al (2004) Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol 53:75–90 10.1016/j.ijpsycho.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ (1994) A twin-family study of alcoholism in women. Am J Psychiatry 151:707–715 [DOI] [PubMed] [Google Scholar]
- Kim U, Wooding S, Ricci D, Jorde LB, Drayna D (2005) Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat 26:199–204 10.1002/humu.20203 [DOI] [PubMed] [Google Scholar]
- Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D (2003) Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 299:1221–1225 10.1126/science.1080190 [DOI] [PubMed] [Google Scholar]
- Li C, Scott LJ, Boehnke M (2004) Assessing whether an allele can account in part for a linkage signal: the Genotype-IBD Sharing Test (GIST). Am J Hum Genet 74:418–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D (1998) Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet 81:216–221 [DOI] [PubMed] [Google Scholar]
- Ma JZ, Zhang D, Dupont RT, Dockter M, Elston RC, Li MD (2003) Mapping susceptibility loci for alcohol consumption using number of grams of alcohol consumed per day as a phenotype measure. BMC Genet Suppl 4:S104 10.1186/1471-2156-4-S1-S104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur JP, Buck LB (2000) A family of candidate taste receptors in human and mouse. Nature 404:601–604 10.1038/35007072 [DOI] [PubMed] [Google Scholar]
- Nurnberger JI Jr, Foroud T, Flury L, Su J, Meyer ET, Hu K, Crowe R, Edenberg H, Goate A, Bierut L, Reich T, Schuckit M, Reich W (2001) Evidence for a locus on chromosome 1 that influences vulnerability to alcoholism and affective disorder. Am J Psychiat 158:718–724 10.1176/appi.ajp.158.5.718 [DOI] [PubMed] [Google Scholar]
- Pickens RW, Svikis DS, McGue M, Lykken DT, Heston LL Clayton PJ (1991) Heterogeneity in the inheritance of alcoholism: a study of male and female twins. Arch Gen Psychiatry 48:19–28 [DOI] [PubMed] [Google Scholar]
- Pronin AN, Tang H, Connor J, Keung W (2004) Identification of ligands for two human bitter T2R receptors. Chem Senses 29:583–593 10.1093/chemse/bjh064 [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H (1998) Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet 81:207–215 [DOI] [PubMed] [Google Scholar]
- Soranzo N, Bufe B, Sabeti PC, Wilson JF, Weale ME, Marguerie R, Meyerhof W, Goldstein DB (2005) Positive selection on a high-sensitivity allele of the human bitter-taste receptor TAS2R16. Curr Biol 15:1257–1265 10.1016/j.cub.2005.06.042 [DOI] [PubMed] [Google Scholar]
- Spielman RS, Ewens, WJ (1996) The TDT and other family-based tests for linkage disequilibrium and association. Am J Hum Genet 59:983–989 [PMC free article] [PubMed] [Google Scholar]
- Tepper BJ (1998) 6-n-Propylthiouracil: a genetic marker for taste, with implications for food preference and dietary habits. Am J Hum Genet 63:1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Ugawa S, Ishida Y, Shibata Y, Murakami S, Shimada S (2001) Identification of coding single-nucleotide polymorphisms in human taste receptor genes involving bitter tasting. Biochem Biophys Res Commun 285:147–151 10.1006/bbrc.2001.5136 [DOI] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, Kwon JM, Wu W, Dick DM, Rice J, Jones K, Nurnberger JI Jr, Tischfield J, Porjesz B, Edenberg HJ, Hesselbrock V, Crowe R, Schuckit M, Begleiter H, Reich T, Goate AM, Bierut LJ (2004) Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet 13:1903–1911 10.1093/hmg/ddh194 [DOI] [PubMed] [Google Scholar]
- Wooding S, Kim UK, Bamshad MJ, Larsen J, Jorde LB, Drayna D (2004) Natural selection and molecular evolution in PTC, a bitter-taste receptor gene. Am J Hum Genet 74:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (1993) International classification of disease, 10th ed. World Health Organization, Geneva, pp 55–59 [Google Scholar]