Abstract
In seven families, six different mutant alleles of TRIOBP on chromosome 22q13 cosegregate with autosomal recessive nonsyndromic deafness. These alleles include four nonsense (Q297X, R788X, R1068X, and R1117X) and two frameshift (D1069fsX1082 and R1078fsX1083) mutations, all located in exon 6 of TRIOBP. There are several alternative splice isoforms of this gene, the longest of which, TRIOBP-6, comprises 23 exons. The linkage interval for the deafness segregating in these families includes DFNB28. Genetic heterogeneity at this locus is suggested by three additional families that show significant evidence of linkage of deafness to markers on chromosome 22q13 but that apparently have no mutations in the TRIOBP gene.
Hereditary hearing loss unaccompanied by other associated clinical features is referred to as “nonsyndromic” and is a genetically heterogeneous neurosensory disorder (Friedman and Griffith 2003; Morton 2004; Smith 2004). We ascertained ∼600 families segregating nonsyndromic deafness. Here, we report 12 of these families, 7 of which are segregating mutant alleles of TRIOBP on chromosome 22q13. The mouse ortholog Triobp (also referred to as “Tara”) encodes a protein that appears to be involved in cytoskeletal organization (Seipel et al. 2001).
After written informed consent was obtained, consanguineous families segregating deafness as an autosomal recessive trait were recruited to our protocols. Approval for this study was obtained from institutional review boards in Pakistan, India, and the United States (at the National Institutes of Health under protocol OH93-N-016). Affected members of 11 of the 12 families reported herein (fig. 1) had prelingual, severe-to-profound hearing loss (audiograms shown in fig. 2) without any obvious associated symptoms or signs, such as dysmorphology, night blindness, vestibular abnormalities, goiter, and renal or cardiac defects, that might represent a syndromic form of deafness (Petit et al. 2001). Physical examinations, including vestibular-function testing, funduscopy, and/or electroretinogram, were conducted on at least two affected individuals from each of these families. Hearing was evaluated using pure-tone audiometry. In family PKDF324, retinitis pigmentosa segregated independently of deafness (data not shown).
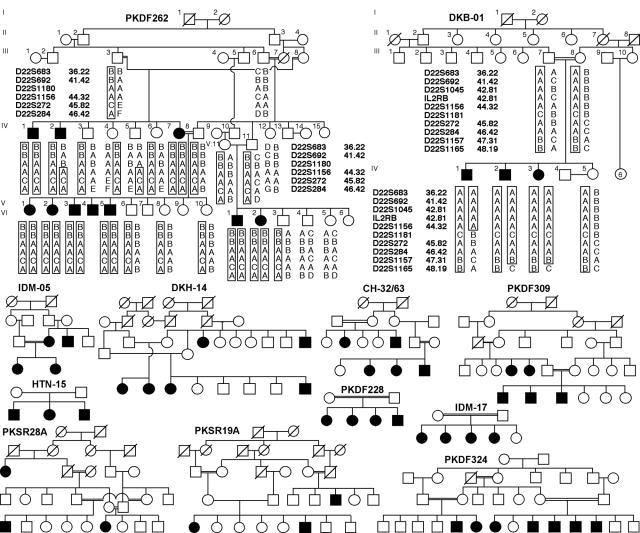
Figure 1.
Pedigrees of 12 consanguineous families from Pakistan and India. Blackened symbols represent individuals with clinically documented congenital deafness. Genotype and haplotype data for families PKDF262 and DKB-01 are shown. For the chromosome 22q13 locus, the haplotype of affected individual IV:2 of family PKDF262 has a proximal breakpoint at D22S1180. Affected individual IV:1 of family DKB-01 has a distal breakpoint at D22S1181, which refines the smallest linkage interval to 702 kb. However, when TRIOBP mutation–containing families are removed, the minimal deafness-linkage interval is ∼10 cM, defined by two of the remaining families, PKSR19A and PKSR28A, with simulated LOD scores of 3.4 and 3.1, respectively (table 1).
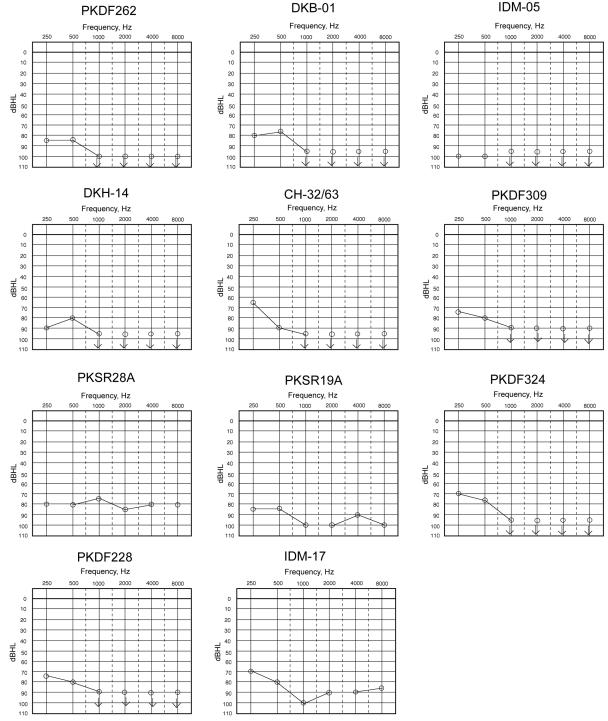
Figure 2.
Audiograms of one congenitally deaf individual from each family. The carriers of a mutant allele have normal hearing.
By use of the Weber 9 marker panel and genomic DNA from affected and unaffected members of family PKSR28A, a genomewide linkage analysis was undertaken after exclusion of linkage of deafness to genetic markers at the known nonsyndromic recessive deafness (DFNB) loci (Hereditary Hearing Loss Homepage). Evidence of linkage of deafness segregating in PKSR28A was detected with marker D22S272 on chromosome 22q13, with a two-point LOD score of 3.1. Haplotype analysis revealed an ∼10-cM region of homozygosity delimited by markers D22S692 (41.42 cM) and D22S274 (51.54 cM). Three STR markers (D22S692, D22S1045, and D22S272) at 22q13 were then used to screen ∼600 families segregating recessive deafness. The deafness phenotype segregating in five additional families from Pakistan and six from India was consistent with linkage to genetic markers in this interval (fig. 1 and table 1). The largest family (PKDF262) (table 1) had a two-point LOD score of 7.2 for D22S272.
Table 1.
Family Data, Mutation of TRIOBP (and Effect on Triobp), and Allele Frequencies[Note]
| Family | Ethnicity | LOD atθ=0 | PathogenicMutation/Polymorphism | Protein Effect | Allele Frequency in Control Population |
| DKB-01 | Indian | 1.8 | 889C→T | Q297X | 0/662 |
| PKDF309 | Pakistani | 3.2 | 2362C→T | R788X | 0/608 |
| PKDF262 | Pakistani | 10.2 | 3202C→T | R1068X | 0/616 |
| HTN15 | Indian | 1.8 | 3202_3203delCG | D1069fsX1082 | 0/616 |
| IDM-05 | Indian | 1.8 | 3225_3226insC | R1078fsX1083 | 0/616 |
| DKH-14 | Indian | 2.9 | 3225_3226insC | R1078fsX1083 | 0/616 |
| CH-32/63 | Indian | 2.2 | 3349C→T | R1117X | 0/616 |
| PKDF324 | Pakistani | 6.3 | None (1193_1195delAAC) | delQ398 | 33/180 |
| PKSR19A | Pakistani | 3.4 | None | … | … |
| PKSR28A | Pakistani | 3.1 | None | … | … |
| IDM-17 | Indian | 1.8 | None | … | … |
| PKDF228 | Pakistani | 1.8 | None | … | … |
Note.— Nucleotide changes are numbered according to the first coding ATG in exon 2 of TRIOBP-6. LOD scores were calculated using parameters described elsewhere (Ahmed et al. 2001)—in brief, the disease was coded as fully penetrant, and the disease-allele frequency was set at 0.001. Meiotic recombination fractions were assumed to be equal for males and females.
Haplotype analysis of affected individuals in all the families was used to refine the genetic interval of this DFNB locus. Family PKDF262 has 10 affected individuals (9 ascertained) in three sibships. Individual IV:2 has a proximal breakpoint for STR marker D22S1180 (fig. 1) at 36164004 bp of the chromosome 22 sequence (UCSC Genome Browser May 2004 assembly). Affected individual IV:1 from family DKB-01 has a distal breakpoint for the STR D22S1181 (fig. 1) at 36866004 bp. Family DKB-01 has only three affected siblings and does not support a statistically significant LOD score (table 1). Nevertheless, we proceeded with the positional cloning of this DFNB gene under the assumption that this small family, which shares a haplotype with a larger family (DKH-14), was segregating a mutation in the same gene responsible for deafness in the other 11 families. Thus, families PKDF262 and DKB-01 led to a tentative refinement of the smallest interval to ∼702 kb on the chromosome 22 physical map, which is gene rich. The linkage interval (figs. 1 and 3) for deafness segregating in all these families overlaps the DFNB28 locus reported elsewhere (Walsh et al. 2000).
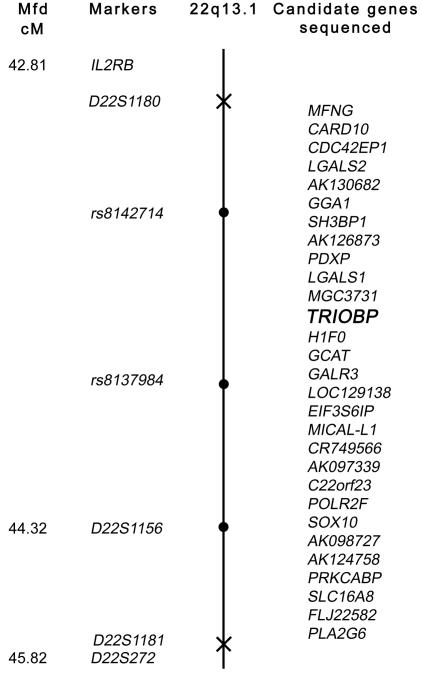
Figure 3.
Genetic map of the 22q13 locus defined by proximal and distal meiotic breakpoints in families PKDF262 and DKB-01, respectively. The Marshfield map distance (Mfd) of polymorphic markers is shown (left) (see Center for Medical Genetics, Marshfield Medical Research Foundation Web site). The proximal breakpoint, D22S1180, and the distal breakpoint, D22S1181, are marked with an X on the chromosome. Linked markers are shown as blackened circles on the chromosome. In two affected individuals from at least six of the families, we evaluated the other 28 annotated genes in this interval for mutations but found none. TRIOBP is shown in bold italics.
There are 29 annotated genes in this 702-kb interval. For all these genes, we sequenced the splice junctions and all coding exons, using DNA from two affected members each from at least six families (fig. 3). Several nucleotide variants were found for many of these genes but were excluded as pathogenic mutations on the basis of >1% frequency in the normal population, as reported in SNP databases, or presence in at least 96 unaffected control individuals from India and Pakistan (information available on request). We also sequenced some of the conserved predicted regulatory elements and all of the exons of SOX10, since mutations of this gene are known to be associated with a syndromic deafness, Waardenburg syndrome type IV (WSIV [MIM 277580]) (Pingault et al. 1998). There is precedence for allelic mutations associated with syndromic and nonsyndromic forms of hereditary deafness (Bork et al. 2001; Petit et al. 2001; Ahmed et al. 2002, 2003). However, we did not find a pathogenic mutation of SOX10.
One of the genes in the chromosome 22q13 interval is TRIOBP (fig. 3). The RefSeq mRNA entry for TRIOBP isoform 1 (TRIOBP-1 [GenBank accession number NM_007032]) has an ORF with a deduced amino acid sequence containing 593 residues (Seipel et al. 2001). However, two other mRNAs for TRIOBP (GenBank accession numbers AB051449 and AK096634) have five and seven additional exons, respectively, that are located upstream of the reported exons and are part of TRIOBP. On the basis of the presence of these additional exons and our evaluation of 5′ and 3′ products of rapid amplification of cDNA ends (RACE) of the mouse ortholog of TRIOBP, a more complete structure of TRIOBP was assembled (fig. 4A). Using cDNA template synthesized from human inner-ear mRNA, we identified additional exons of TRIOBP. There are also predicted exons of TRIOBP based on the annotation in the UCSC database.
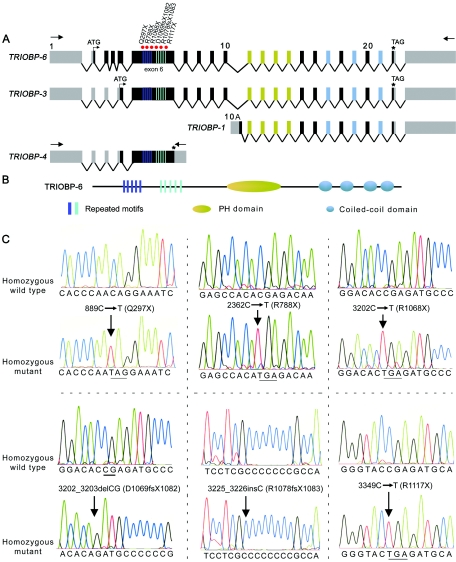
Figure 4.
Structure and mutations of TRIOBP associated with deafness. A, Schematic representation of the genomic structure of isoforms of human TRIOBP and the locations of the mutations in exon 6 associated with deafness. Arrows represent the location of the primers used for RT-PCR. Red dots indicate the site of the mutations in exon 6 of TRIOBP. B, Protein structure of TRIOBP-6. There are five copies each of two different repeated motifs (dark blue and light blue rectangles) that have the amino acid sequences TPCA/I/TQR/WDNPRASSPNRT/ST/AQRDN/SPR and VCIGHRDAPRAS/TS/FPP. C, Mutations of TRIOBP segregating in six families. Electropherograms of amplimers from genomic DNA templates illustrate homozygosity for mutations found in all affected family members and homozygosity for the wild-type allele in an unaffected individual. All obligate carriers are heterozygous (not shown). All of the mutations described here are numbered beginning with +1 at the A base of the translation start codon (ATG) in exon 2 of TRIOBP-6. The stop codons due to nonsense mutations are underlined.
We describe here three additional isoforms of human TRIOBP (TRIOBP-3, TRIOBP-4, and TRIOBP-6 [GenBank accession numbers DQ228003, DQ228004, and DQ228005, respectively]) (fig. 4A). TRIOBP-6 is the longest isoform and has 23 exons (fig. 4A), of which 21 are coding. This protein has 2,365 aa residues (261 kDa). TRIOBP-3 and TRIOBP-4 are shorter transcripts, encoding 2,193 and 1,144 aa residues, respectively. Depending on the isoform, TRIOBP has five copies each of two repeated motifs encoded by exon 6, a predicted pleckstrin homology (PH) domain, and four coiled-coil regions (SMART [Schultz et al. 1998]) (fig. 4B).
We sequenced all the exons of TRIOBP (fig. 4A) in genomic DNA of affected persons from each of the 12 families (fig. 1). All exons were amplified by PCR from genomic DNA in a 20-μl reaction volume, with primers flanking all the exon-intron boundaries (table 2) as described elsewhere (Ahmed et al. 2001). We identified six mutations (fig. 4C and table 1), including four nonsense mutations (Q297X, R788X, R1068X, and R1117X) and two frameshift mutations (D1069fsX1082 and R1078fsX1083) (table 1). All these mutations were located in exon 6 of TRIOBP, and all six alleles result in truncations of the protein. Exon 6 is 3.319 kb and encodes 1,106 aa. None of these truncating mutations of TRIOBP was detected in ∼300 DNA samples (table 1) from unaffected Pakistani, Indian, and North American control individuals.
Table 2.
Primers Used to Sequence 24 Exons of TRIOBP and for Cloning Full-Length Isoforms and RT-PCR[Note]
|
Primer Sequence(5′→3′) |
|||
| Use for Primerand Exon(s) or Isoform | Forward | Reverse | Product(bp) |
| Sequencing of exons: | |||
| 1 | GAGGGATCCGGGGAGGAA | GGAGTGGAGCTGGGTCAGGA | 695 |
| 2 | ATTTCCTTGGTCCTTGTCTGGAAAC | GAAGTCCAGCATCTGGGTTCAACT | 382 |
| 3 | CCGAGGAGATGCCTGGAGACT | ATGCCAGCAGCCATTAGAGACAG | 356 |
| 4 | CTCTCACCATTGGCTCCCTTT | CTGTGCAGAAATCCCCACTCTC | 523 |
| 5 | TCTGCCCCTCTCATTTGGAGAGTAT | TAACACTCAGGCCACCAAAGTAAGC | 368 |
| 6 | AGTGAGACCTGTAGGAGGAGGGTGT | CCACACACACACACAAAACAGAAAA | 3,641 |
| 7 | CTGTTGGAGGTGGGAGCAGAG | CATAGAGGACCTTCCTGTCCAAGC | 300 |
| 8a | CAGCCAGGTCCCCAGAGAGAG | GCTCTCCACATCCCCACCAG | 383 |
| 8b | CCAGGGCCCTCATAGACACCTAGAA | CCCAGACCCCCTCTCCTGGT | 416 |
| 8c | AGCTTGACTGGAGGGATCTGCTT | AGTGGGGCTGACTTTCTGGCTAC | 489 |
| 9 | ATAGAATGGATGGATGAGGTGGACA | CTAGGGCTCTTCTGAGGAATGAAGC | 250 |
| 10 | AATGGGCAGGGGAGGCTGTAG | CTCCCTCCCATCCCCGTCTCTAT | 281 |
| 10A | CCGCTGTTTGTCTCGGCTTC | CTCTCGCCTTCGGGAAATGAG | 382 |
| 11 | CCACAGGACTTGGGGACAGG | AGCAGCACAAGCCAGCTTCC | 203 |
| 12 and 13 | AGGCTCAGAGAGGAAATGGAG | GTGGATCTGGAAGCCATAGTTG | 463 |
| 14 | CACTTAGCATATCTGGGGGAGA | CTCTTCCCATGTTGTTGACAGA | 444 |
| 15a | AGGGGTGAGGACCCACCTGA | CTGTCTGTGGCCTCAAACCACTTG | 339 |
| 15b | ACCCCAGCCCGCACTCCTGAC | CAGGGGTGAGCTCCCCATCACTGTA | 448 |
| 16 | TCTCAAACCCTGAGGCATTC | GGGGAGAGTAAATGAGATGACG | 402 |
| 17 | CTCCTCTGGAGGCTGGGGAAG | CCAGTTTGGCTAGGAGGAACCTCAG | 297 |
| 18 | GTGCTCTTTGCATAGCACTCCT | ACCTTCTGCTTCTGTTTCTTGC | 416 |
| 19 and 20 | GAGTCCTGGGATGCTTTAAGTC | CTTTCCTGCCACAGGTGATAC | 540 |
| 21 | AGATAGGGGCTGCATTCTAGG | AGAGCCTGAGGAAGGATGAAG | 318 |
| 22 | AGGGATGAAGCGAGGTATCTG | CTTTTTACAGAACGGGAATTGG | 406 |
| 23 | CTGAGCTGCTTGGGAAGGA | GGCCACCAGCTCAGAAGC | 598 |
| Cloning of full-length isoforms: | |||
| TRIOBP-6/3 | TCATAGGAACTGCCCTGGCCTGACT | GCACGTGTGTGCGTATGTGTCTGTG | 7,113 |
| TRIOBP-4 | CCAAGATTGGCCACAAAAGCCTGAT | GTCCTGCTGCCGAGCCTCCTC | 3,901 |
| Triobp-1 | GGAAGGGAAGGGCTGGAGCTACG | GGGTGACTCACGGAACAGAAGAG AAGTG | 2,492 |
| Triobp-3 | AGTCGCACACTGACCATGCAAATGA | ACGAGCTCAGATCCGCAGTGTCTGT | 6,801 |
| Triobp-4 | AGTCGCACACTGACCATGCAAATGA | GGGAAGTTAAATTGCTTTAATCAGGT ATCACAGGA | 3,740 |
| Triobp-5 | AGTCGCACACTGACCATGCAAATGA | ACGAGCTCAGATCCGCAGTGTCTGT | 6,663 |
| RT-PCR: | |||
| Triobp-3 | ACCTGAGGATGGGGCCTGGAC | CGCTGCAGCTGCTCCATCACC | 1,635 |
| Triobp-4 | CTCAGAGGGTCTGGTCCCCAACAAC | GCTCCTGTGTTTTTGACTACCTGG CTA | 2,870 |
| Triobp-5 | ACCTGAGGATGGGGCCTGGAC | CGCTGCAGCTGCTCCATCACC | 1,496 |
Note.— All the primers were designed using Primer3.
No mutations of TRIOBP were identified in affected individuals from families PKDF324, PKSR19A, PKSR28A, IDM-17, and PKDF228. Three of these five families have significant LOD scores (table 1) indicating linkage of deafness to chromosome 22q13 markers, which suggests the presence of additional unknown exons of TRIOBP, undetected mutations, or locus heterogeneity.
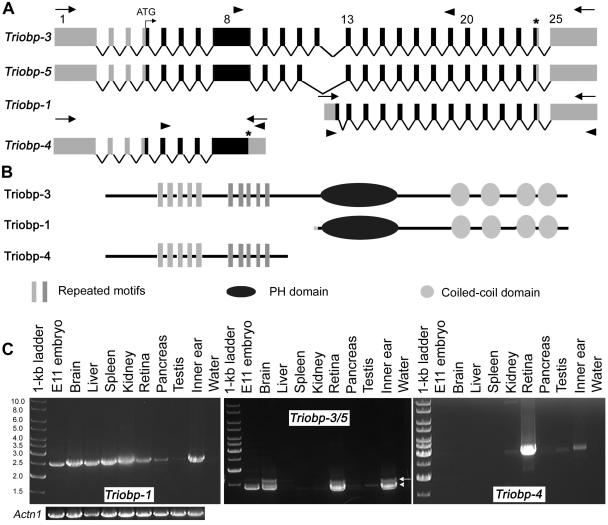
From the mouse inner-ear cDNA library, we found previously reported short isoforms (Triobp-1 and Triobp-2) and three additional isoforms (Triobp-3, Triobp-4, and Triobp-5 [GenBank accession numbers DQ228000, DQ228002, and DQ228001, respectively]) (fig. 5). The longest isoform (Triobp-3) has 25 exons, of which 21 are coding and would produce a protein of 2,014 aa (224 kDa) that is 65% identical to human TRIOBP-3 (fig. 5A and 5B). Four consecutive exons of mouse Triobp-3 (exons 5–8) correspond to the sequence encoded by exon 6 (3,319 bp) of human TRIOBP. In addition to characterizing the isoforms of Triobp, we also determined the expression pattern of Triobp isoforms in various tissues. Triobp-1 is widely expressed (fig. 5C), as reported elsewhere (Seipel et al. 2001), whereas other isoforms have a more limited pattern of expression (fig. 5C). Triobp-3 is present in the brain, liver, kidney, retina, and inner ear, whereas Triobp-5 is found in the whole embryo, brain, kidney, retina, pancreas, and inner ear (fig. 5C). Triobp-4 seems to be expressed in the kidney, retina, testis, and inner ear (fig. 5C).
Figure 5.
Genomic structure of Triobp mouse isoforms and their expression in various tissues. A, Triobp splice variants. There are at least five different splice variants present in the mouse inner ear, including the previously reported short isoforms (Triobp-1 and Triobp-2). Locations of the primers used for RT-PCR expression profile are shown as arrowheads. B, Predicted protein products of different Triobp splice variants. Triobp-4 does not encode the PH domain and the coiled-coil regions. Triobp-1 encodes only the PH domain and the four coiled-coil domains. C, Expression profiles of Triobp-1, Triobp-3/5, and Triobp-4. PCR primers (table 2), shown as arrowheads in panel A, were designed to amplify each isoform from cDNA prepared from different mouse tissues. Left profile, Triobp-1 is widely expressed, whereas other isoforms have a more limited pattern of expression. Triobp-3 (arrow) is present in the brain, liver (faint band), kidney (faint band), retina (P15), and inner ear (P1–P5). Middle profile,Triobp-5 (arrowhead) is found in mouse embryo cDNA (E11.5), brain, kidney (faint band), retina, pancreas (faint band), and inner ear. Right profile, Triobp-4 seems to be expressed in kidney (faint band), retina, testis (faint band), and inner ear. These transcripts do not provide an exhaustive list of the isoforms of Triobp. A smaller, uncharacterized cDNA is present in many tissues (middle profile). There are 33 inner-ear RIKEN ESTs for Triobp (168–488 bp; not shown). PCR amplification of Actn1 cDNA encoding α-actinin was used as a control for the quality and quantity of RT-PCR templates.
Relatively little is know about the function of Triobp. In a yeast two-hybrid screen, a Triobp isoform equivalent to Triobp-1 (fig. 5B) was identified as a protein that interacts with Trio, which is a member of a large family of Dbl homology guanine nucleotide exchange factors (DH-GEFs). DH-GEFs are involved in activating Rho guanosine triphosphatases (Rossman et al. 2005), which mediate a variety of cellular functions, including actin remodeling (Rossman et al. 2005). Triobp is also reported to have filamentous actin (F-actin) bundling activity on the basis of an in vitro binding assay in HeLa cells (Seipel et al. 2001). Moreover, Triobp-1 stabilizes F-actin structures, as indicated by the relative resistance of Triobp-1–expressing cells to latrunculin B, an F-actin destabilizer (Seipel et al. 2001). Perhaps in the inner ear, the function of Triobp-1 is important for formation or stabilization of the cytoskeletal structure of stereocilia and/or the cuticular plate, both actin-rich structures in sensory hair cells (Frolenkov et al. 2004). However, all six mutations causing deafness in humans are located in exon 6 of TRIOBP (equivalent to exons 5–8 of mouse Triobp), which encodes an amino acid sequence that is not present in Triobp-1 (fig. 5). Given the complex alternative splicing of this gene, it appears that a variety of isoform-specific mutations of Triobp and antisera to Triobp will be essential to understanding the normal function of this gene in the auditory system.
Acknowledgments
We are indebted to participating family members and to Dennis Drayna, Andrew Griffith, Shin-ichiro Kitajiri, and Julie Schultz, for their critiques of our manuscript, and Ayala Lagziel, for RACE-ready cDNA from mouse inner ear. Research in Pakistan was funded by the Higher Education Commission and the Ministry of Science and Technology at Islamabad, Pakistan. This study was supported by the National Institutes of Health Intramural Research programs of the National Human Genome Research Institute and by National Institute on Deafness and Other Communication Disorders grants ZO1 DC000035-07 and ZO1 DC000039-07.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics/
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for TRIOBP1 [accession number NM_007032], mRNAs for TRIOBP [accession numbers AB051449 and AK096634], TRIOBP-6 [accession number DQ228005], TRIOBP-3 [accession number DQ228003], TRIOBP-4 [accession number DQ228004], Triobp-3 [accession number DQ228000], Triobp-4 [accession number DQ228002], and Triobp-5 [accession number DQ228001])
- Hereditary Hearing Loss Homepage, http://webhost.ua.ac.be/hhh/
- Online Mendelian Inheritance of Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for WSIV)
- Primer3, http://www.genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi
- UCSC Genome Browser May 2004 assembly, http://genome.ucsc.edu/cgi-bin/hgGateway
References
- Ahmed ZM, Riazuddin S, Ahmad J, Bernstein SL, Guo Y, Sabar MF, Sieving P, Riazuddin S, Griffith AJ, Friedman TB, Belyantseva IA, Wilcox ER (2003) PCDH15 is expressed in the neurosensory epithelium of the eye and ear and mutant alleles are responsible for both USH1F and DFNB23. Hum Mol Genet 12:3215–3223 10.1093/hmg/ddg358 [DOI] [PubMed] [Google Scholar]
- Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, Morell RJ, Friedman TB, Riazuddin S, Wilcox ER (2001) Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet 69:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Smith TN, Riazuddin S, Makishima T, Ghosh M, Bokhari S, Menon PS, Deshmukh D, Griffith AJ, Riazuddin S, Friedman TB, Wilcox ER (2002) Nonsyndromic recessive deafness DFNB18 and Usher syndrome type IC are allelic mutations of USHIC. Hum Genet 110:527–531 10.1007/s00439-002-0732-4 [DOI] [PubMed] [Google Scholar]
- Bork JM, Peters LM, Riazuddin S, Bernstein SL, Ahmed ZM, Ness SL, Polomeno R, et al (2001) Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet 68:26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman TB, Griffith AJ (2003) Human nonsyndromic sensorineural deafness. Annu Rev Genomics Hum Genet 4:341–402 10.1146/annurev.genom.4.070802.110347 [DOI] [PubMed] [Google Scholar]
- Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ (2004) Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet 5:489–498 10.1038/nrg1377 [DOI] [PubMed] [Google Scholar]
- Morton CC (2004) Gene discovery in the auditory system using a tissue specific approach. Am J Med Genet A 130:26–28 10.1002/ajmg.a.30049 [DOI] [PubMed] [Google Scholar]
- Petit C, Levilliers J, Hardelin JP (2001) Molecular genetics of hearing loss. Annu Rev Genet 35:589–646 10.1146/annurev.genet.35.102401.091224 [DOI] [PubMed] [Google Scholar]
- Pingault V, Bondurand N, Kuhlbrodt K, Goerich DE, Prehu MO, Puliti A, Herbarth B, Hermans-Borgmeyer I, Legius E, Matthijs G, Amiel J, Lyonnet S, Ceccherini I, Romeo G, Smith JC, Read AP, Wegner M, Goossens M (1998) SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat Genet 18:171–173 10.1038/ng0298-171 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6:167–180 10.1038/nrm1587 [DOI] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA 95:5857–5864 10.1073/pnas.95.11.5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seipel K, O’Brien SP, Iannotti E, Medley QG, Streuli M (2001) Tara, a novel F-actin binding protein, associates with the Trio guanine nucleotide exchange factor and regulates actin cytoskeletal organization. J Cell Sci 114:389–399 [DOI] [PubMed] [Google Scholar]
- Smith RJ (2004) Clinical application of genetic testing for deafness. Am J Med Genet A 130:8–12 10.1002/ajmg.a.30053 [DOI] [PubMed] [Google Scholar]
- Walsh TD, Shahin H, Morrow J, King M-C, Lynch E, Avraham K, Kanaan M (2000) DFNB28, a novel locus for prelingual nonsyndromic autosomal recessive hearing loss, maps to 22q13 in a large consanguineous Palestinian kindred. Paper presented at the NIDCD Hereditary Hearing Impairment Consortium, Philadelphia, October 4 [Google Scholar]