Abstract
In a large consanguineous Palestinian kindred, we previously mapped DFNB28—a locus associated with recessively inherited, prelingual, profound sensorineural hearing impairment—to chromosome 22q13.1. We report here that mutations in a novel 218-kDa isoform of TRIOBP (TRIO and filamentous actin [F-actin] binding protein) are associated with DFNB28 hearing loss in a total of nine Palestinian families. Two nonsense mutations (R347X and Q581X) truncate the protein, and a potentially deleterious missense mutation (G1019R) occurs in a conserved motif in a putative SH3-binding domain. In seven families, 27 deaf individuals are homozygous for one of the nonsense mutations; in two other families, 3 deaf individuals are compound heterozygous for the two nonsense mutations or for Q581X and G1019R. The novel long isoform of TRIOBP has a restricted expression profile, including cochlea, retina, and fetal brain, whereas the original short isoform is widely expressed. Antibodies to TRIOBP reveal expression in sensory cells of the inner ear and colocalization with F-actin along the length of the stereocilia.
Approximately 1 in 1,000 children in the United States are born with hearing loss, and ∼60% of these cases are due to genetic factors (Marazita et al. 1993). The genetic causes of hearing loss are highly heterogeneous: 89 loci for hereditary hearing loss have been mapped, and 45 of the corresponding genes have been cloned (Hereditary Hearing Loss Homepage). Many of these genes were first identified in extended consanguineous kindreds. In particular, studies in the Palestinian population, which has a long historical tradition of endogamous marriage, provided the first clues to the identification of TMPRSS3 (Bonne-Tamir et al. 1996; Scott et al. 2001), stereocilin (Campbell et al. 1997; Verpy et al. 2001), otoancorin (Zwaenepoel et al. 2002), whirlin (Mustapha et al. 2002; Mburu et al. 2003), and DFNB33 (Medlej-Hashim et al. 2002). In addition, pendrin (Baldwin et al. 1995) and DFNB17 (Greinwald et al. 1998) were first mapped in Palestinian Druze families.
To identify novel genes for hereditary hearing loss, we ascertained children with prelingual hearing loss from Palestinian schools for the deaf. Thus far, 156 families have been enrolled in the study. One of these, family K, is an Orthodox Christian family that traces its ancestry since the mid-eighteenth century to the area of Bethlehem and Beit Sahour. Pure-tone audiometry and vision tests were performed at audiology centers in the Palestinian Authority. Hearing loss in family K was sensorineural, bilateral, symmetrical, and profound; the mode of inheritance appeared to be recessive. No signs of mixed hearing loss were noted, and vision was normal. Blood samples were drawn from participating individuals after informed consent was obtained in accordance with the guidelines of the Bethlehem University Research Committee, the Tel Aviv University Helsinki Committee, and the University of Washington Human Subjects Division.
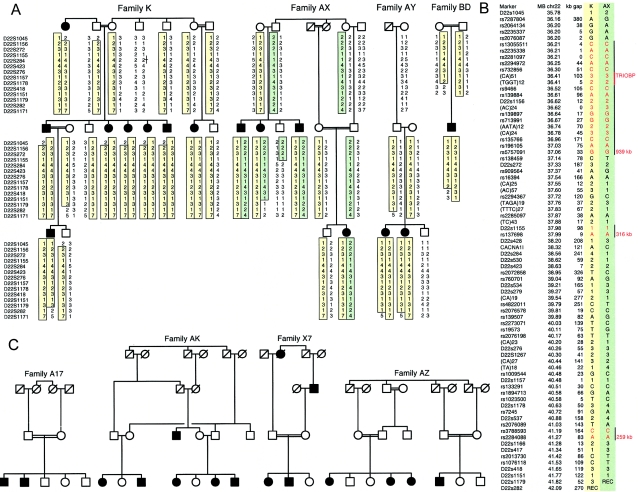
To map the gene for hearing loss in family K, we performed genomewide linkage analysis with DNA from 18 informative relatives, using 379 markers from ABI PRISM Linkage Mapping Set 2 (Applied Biosystems). Allele sizes were determined using ABI GENESCAN version 2.02 software with manual review. Software developed in our laboratories displays genotypes for pedigrees and collects data from automated genome scans for linkage analysis by FASTLINK version 3.0 (Lathrop et al. 1984) and LINKAGE version 5.1 (Cottingham et al. 1993). The genomewide scan yielded a maximum LOD score of 4.19 for linkage of hereditary hearing impairment to marker D22S423 on chromosome 22q13.1. Fine mapping with additional microsatellite markers defined linkage to inherited hearing loss in family K at the 6.3-Mb region bounded by D22S1045 and D22S282 (fig. 1A). We named this locus “DFNB28” (Walsh et al. 2000).
Figure 1.
Palestinian families with hereditary hearing loss linked to 6.3 Mb on chromosome 22q13 (DFNB28). A, Families K, AX, AY, and BD. In these families, hearing loss is linked to two haplotypes, shown in yellow and green. The order of markers was determined from the genomic sequence of chromosome 22 (UCSC Genome Browser May 2004 assembly). B, Homozygosity mapping based on 76 SNPs and microsatellite markers. Genotypes in red are shared by the yellow and green haplotypes. Black vertical lines indicate regions of 939 kb, 316 kb, and 259 kb that were identical either by descent or by state in all affected individuals. The position of TRIOBP in the 939-kb homozygous region is indicated. C, Families A17, AK, X7, and AZ. In these families, hearing loss was also linked to markers at DFNB28.
To further constrain the region of linkage, we mapped markers from the D22S1045–D22S282 interval in other Palestinian kindreds with prelingual, profound hearing loss. In three apparently unrelated families—AX, AY, and BD—linkage of hearing loss was consistent with DFNB28 (fig. 1A). Families K, AX, AY, and BD were not aware of any biological relationship to one another but were from the same small geographic area and were part of the same Orthodox Christian community, which suggests an ancestral relationship. We tested homozygosity in the D22S1045–D22S282 interval among deaf individuals in these four families, using 76 informative SNPs and microsatellite markers (fig. 1B). Regions of 939 kb, 316 kb, and 259 kb were homozygous either by descent or by state in all individuals with hearing loss. These three regions of homozygosity harbored 34 known or hypothetical genes (UCSC Genome Browser May 2004 assembly). We sequenced all known exons, flanking splice junctions, and the 5′-UTR and 3′-UTR regions of these genes and found no variants likely to be pathogenic. (A list of variant sites is available on request.)
The homozygous region of 939 kb (fig. 1B) harbored the gene encoding TRIOBP (also known as “TARA” or “HRIHFB2122”), a filamentous actin (F-actin) binding protein that associates with the TRIO guanine nucleotide exchange factor and regulates actin cytoskeleton organization (Seipel et al. 2001). TRIOBP was an excellent gene candidate for DFNB28 hearing loss, because of both its position and its function. However, the known exons of TRIOBP yielded no likely pathogenic mutations in families K, AX, AY, and BD. Therefore, we used information from databases to explore whether additional, previously undetected alternate transcripts of TRIOBP might be hidden in incompletely annotated genomic regions.
The UCSC Human Genome Browser May 2004 assembly includes as known genes two isoforms of TRIOBP that share the same translation start site at 22:36466921 and the surrounding CpG island. In addition, two transcripts listed in GenBank, AB051449 from brain and AK096634 from fetal brain, share some exons with TRIOBP and include additional exons that extend the locus 50 kb upstream of the known TRIOBP sequence. However, AB051449 and AK096634 differ from one another and from both annotated TRIOBP sequences. Neither AB051449 nor AK096634 includes a likely translation start site or maps near a CpG island. The closest CpG island to the uncharacterized transcripts lies at the 5′ end of another uncharacterized transcript, AL160132, which suggests that the complete TRIOBP locus might encompass 86 kb of genomic sequence rather than 28 kb, the size of original TRIOBP. In contrast to the human sequence, mouse sequence from the UCSC Genome Browser March 2005 assembly did not include any transcripts suggesting longer isoforms of TRIOBP. However, comparison of sequence on human chromosome 22 and syntenic mouse chromosome 15 indicated highly conserved sequence at multiple sites in the hypothetical 86-kb TRIOBP locus. We hypothesized that these conserved regions might include exons corresponding to long isoforms of TRIOBP in both human and mouse and that some of these isoforms might be expressed in cochlea.
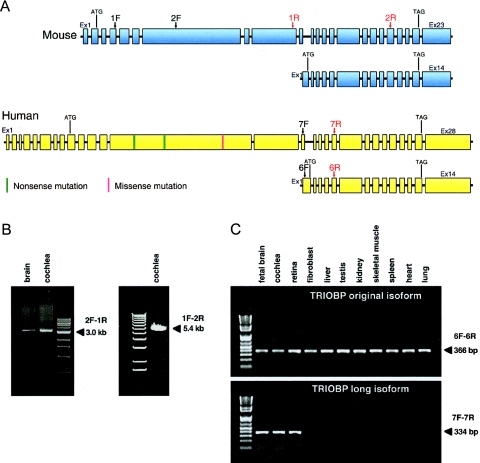
To identify exons that were part of longer TRIOBP transcripts, we undertook several experiments. We first generated cDNA specific to TRIOBP by reverse transcription of total RNA from mouse cochlea, mouse brain, and human fetal brain, using primers matching the 3′ UTR of human and mouse original TRIOBP (table 1). We then used sequences of AB051449, AK096634, and AL160132 and orthologous sequence in mouse to design primer pairs for amplification of mouse and human TRIOBP-specific cDNAs (table 1). We sequenced the products, aligned traces to mouse and human genomes by BLAT, and annotated the exons (fig. 2A and 2B). Interestingly, experimentally determined sequence from human fetal brain differed in exon structure from all of AB051449, AK096634, and AL160132, suggesting multiple alternative transcripts of the long isoform.
Table 1.
RT-PCR Primers for TRIOBP
| Use for Primerand Primer | Primer Sequence(5′→3′) | Locationa | GenBankSequence | ||
| Generate TARA -specific cDNA and determine gene structureb: | |||||
| RT primer, human | GCAAGGTGAGACCCAGTGCGAGG | 8056–8034 | DQ278603 | ||
| RT primer, mouse | GCAAGGTAAAGACCCTGTGCAAGG |
6408–6385 |
DQ278602 | ||
| Forward |
Reverse |
Forward |
Reverse |
||
| cDNA pair, human: | |||||
| H1 | AAGCGAGATGGTGACGACCG | AGCCTTGCCCAGTGCTGTCC | 1–20 | 1631–1612 | |
| H2 | TCCGCCACCCCTGATGATAC | GAGGTCTCTGTGTCCGCAGC | 1375–1394 | 5077–5058 | |
| H3 | GGAGACCAGGCACAACTTGG | CTGCTCAGGGACATCGTTTG | 4734–4753 | 5712–5693 | |
| H4 | CCCAGTCAATGGACACAGCC | GCTCCTGCCACTTCTTCTCG | 5751–5770 | 6757–6738 | |
| H5 | ACTGAGGACCAGCAAAACCG | ACAGAGGGTGGGGAGAAAGC | 6703–6722 | 7857–7838 | DQ278603 |
| cDNA pair, mouse: | |||||
| M1 | AGGAGCCTCACAGCCCTTC | TCCGCTTGTCACTTTGGTG | 353–371 | 4251–4233 | |
| M2 | AGGGATGTTTTCCAGGCTGC | GCTCTTCTGCCTGCCTTGTC | 1039–1058 | 5722–5703 | |
| M3 | GTGCCCCCCTGACTGATGAC | GCCTTTTCCCTCTGGTTGAC | 5075–5094 | 6635–6616 | |
| M4 | CTGTGGCTTCCCTGCTATGG | CGCTGGCTGGTTCTGTTGAG | 125–144 | 1325–1306 | DQ278602 |
| Evaluate expression in human tissuesc: | |||||
| cDNA pair: | |||||
| H6 | GTTCCTGCCTCCTGCTCCCG | GAGCCTCGATCCAGTTCCGC | 128–147 | 493–474 | AF281030 |
| H7 | CAGAAGCAGGCAGACTCG | CTGGGGCTGAAGTTGGAC | 6007–6024 | 6340–6323 | DQ27803 |
Figure 2.
Determination of the genomic structure of isoforms of TRIOBP. A, Mouse and human TRIOBP isoforms amplified from cDNA derived from mouse cochlea and human fetal brain. RT-PCR primer pairs 1F-2R and 2F-1R were used to determine the mouse long isoform of TRIOBP. RT-PCR primers H1-H5 (table 1; not shown in this fig.) were used to determine the human long isoform of TRIOBP. RT-PCR primers 6F-6R and 7F-7R were used to test expression in multiple human tissues. B, Mouse cochlear and brain cDNA amplified by RT-PCR. Products were electrophoresed on agarose gels alongside a molecular-weight size marker. C, Expression of short and long transcripts of TRIOBP in human tissues. Human adult cochlear total RNA was obtained from Phylogeny and fibroblast RNA was a gift from Peter Byers (University of Washington). All other RNAs were purchased from Clontech. Total RNA (5 μg) was reverse transcribed with a primer located in the shared 3′ UTR of both isoforms (table 1). cDNA was amplified for 30 cycles with primers 6F-6R and 7F-7R, specific to each isoform (table 1), and products were electrophoresed on an agarose gel alongside a molecular-weight size marker. The original TRIOBP isoform is widely expressed in all tissues tested. The novel long isoform is expressed only in fetal brain, cochlea, and retina. Ex = exon.
From mouse cochlea, the ORF of the longest isoform of TRIOBP from cochlea comprises 5,907 bp in 21 coding exons, encoding a predicted 219-kDa protein of 1,969 aa (GenBank accession number DQ278602) (fig. 2A and table 2). From human fetal brain, the ORF of the longest isoform of TRIOBP comprises 6,801 bp in 19 coding exons, encoding a predicted 250-kDa protein of 2,267 aa (GenBank accession number DQ278603) (fig. 2A and table 3). Remarkably, the human fetal brain transcript includes eight exons in the 5′ UTR (table 3), of which six (exons 1–6) are an ORF potentially encoding 213 aa, which raises the possibility of still other translation start sites associated with alternative splicing.
Table 2.
Exons of Mouse TRIOBP Isoforms Aligned to UCSC Genome Browser March 2005 Assembly, Chromosome 15
|
Position |
||||
| Start | End | Exon in Long Isoform of TRIOBP | Exon in Original TRIOBP | Primer Sites |
| 78999524 | 78999625 | 1 | ||
| 79002191 | 79002342a | 2 | ||
| 79008392 | 79008491 | 3 | ||
| 79009514 | 79009659 | 4 | 1F | |
| 79011142 | 79011361 | 5 | ||
| 79012236 | 79012392 | 6 | ||
| 79017581 | 79019873 | 7 | 2F | |
| 79023822 | 79023933 | 8 | ||
| 79024539 | 79025579 | 9 | 1R | |
| 79027754 | 79027831 | 10 | ||
| 79034359 | 79034495b | 1 | ||
| 79039179 | 79039235 | 11 | 2 | |
| 79042104 | 79042172 | 12 | 3 | |
| 79042272 | 79042361 | 13 | 4 | |
| 79042616 | 79042725 | 14 | 5 | |
| 79044191 | 79044719 | 15 | 6 | |
| 79045329 | 79045439 | 16 | 7 | |
| 79051084 | 79051231 | 17 | 8 | |
| 79052808 | 79052910 | 18 | 9 | |
| 79053335 | 79053494 | 19 | 10 | 2R |
| 79053572 | 79053685 | 20 | 11 | |
| 79054993 | 79055079 | 21 | 12 | |
| 79055732 | 79055892c | 22 | 13 | |
| 79056489 | 79057154 | 23 | 14 | |
For the long isoform of TRIOBP, ATG is located at position 79002319.
For original TRIOBP, ATG is located at position 79034490.
For both the long isoform and original TRIOBP, TAG is located at position 79055889.
Table 3.
Exons of Human TRIOBP Isoforms Aligned to UCSC Genome Browser May 2004 Assembly, Chromosome 22
|
Position |
||||
| Start | End | Exon in Long Isoform of TRIOBP | Exon in Original TRIOBP | Primer Sites |
| 36406921 | 36406982 | 1 | ||
| 36408417 | 36408522 | 2 | ||
| 36408819 | 36408867 | 3 | ||
| 36409357 | 36409499 | 4 | ||
| 36411204 | 36411301 | 5 | ||
| 36411681 | 36411931 | 6 | ||
| 36413839 | 36413917 | 7 | ||
| 36418095 | 36418203 | 8 | ||
| 36421813 | 36421986a | 9 | ||
| 36430934 | 36431073 | 10 | ||
| 36433717 | 36433918 | 11 | ||
| 36436270 | 36436441 | 12 | ||
| 36443692 | 36447010 | 13 | ||
| 36454908 | 36455949 | 14 | ||
| 36459149 | 36459226 | 15 | 7F | |
| 36466741 | 36466927b | 1 | 6F | |
| 36472279 | 36472335 | 16 | 2 | |
| 36475423 | 36475491 | 17 | 3 | |
| 36475608 | 36475697 | 18 | 4 | |
| 36476057 | 36476163 | 19 | 5 | 6R, 7R |
| 36478120 | 36478645 | 20 | 6 | |
| 36479661 | 36479771 | 21 | 7 | |
| 36486177 | 36486324 | 22 | 8 | |
| 36488581 | 36488683 | 23 | 9 | |
| 36489535 | 36489694 | 24 | 10 | |
| 36489769 | 36489882 | 25 | 11 | |
| 36492157 | 36492243 | 26 | 12 | |
| 36493108 | 36493271c | 27 | 13 | |
| 36494290 | 36495347 | 28 | 14 | |
For the long isoform of TRIOBP, ATG is located at position 36421873.
For original TRIOBP, ATG is located at position 36466921.
For both the long isoform and original TRIOBP, TAG is located at position 36493269.
Reading frames of the long and short isoforms of TRIOBP are the same. Independent transcription and translation of the short isoform are maintained because both mouse and human long isoforms skip exon 1 of the short isoform, which contains an alternate 5′ UTR and 2 aa (fig. 2A). Predicted human and mouse protein sequences specific to the long isoform of TRIOBP (human aa 31–1689 and mouse aa 34–1390) are 56% identical; human and mouse protein sequences of original TRIOBP (human aa 1690–2267 and mouse aa 1391–1969) are 88% identical. In the large central exon of the long isoform (fig. 2A), the human transcript includes more replicates of a repeated motif than does the mouse transcript, leading to a predicted protein in human comprising 298 more amino acid residues than the predicted protein in mouse.
To evaluate expression of the short and long isoforms of TRIOBP, we performed RT-PCR in 11 human tissues (fig. 2C). Primer pairs were specific to each isoform (table 1); in particular, the forward primer for the short isoform was located in original TRIOBP exon 1, which is skipped in the long isoform. The short transcript was highly expressed in all tissues tested. The long transcript was expressed in fetal brain, retina, and cochlea but was not detectable in the other tissues (fig. 2C).
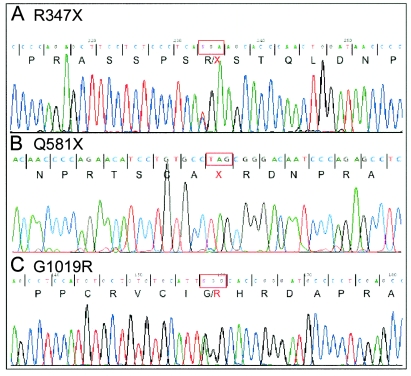
Expression of the long TRIOBP transcript, which was restricted but included cochlea, suggested that it might be a candidate gene for DFNB28 hearing loss. Using genomic DNA of deaf individuals and obligate carriers from families K, AX, AY, and BD (fig. 1A), we sequenced each newly identified exon of the long TRIOBP isoform. We identified a previously unreported substitution, 1039C→T, in human coding exon 5 that was homozygous in all deaf relatives in families K, AX, AY, and BD and heterozygous in obligate carriers (fig. 3A). Predicted translation of human cochlear and fetal brain transcripts indicated that 1039C→T would yield nonsense mutation R347X. The orthologous mouse substitution would also create a stop codon.
Figure 3.
Sequences of mutations in the long isoform of TRIOBP associated with DFNB28 hearing loss. Amino acids are indicated below the nucleotide sequences; mutant codons are indicated by red boxes. A, Sequence from a hearing individual from family K who is heterozygous for 1039C→T (R347X). B, Sequence from a deaf individual from family AK who is homozygous for 1741C→T (Q581X). C, Sequence from a deaf child who is compound heterozygous for 3055G→A (G1019R) and R347X.
In parallel, we tested other Palestinian families for linkage of hearing loss to the DFNB28 locus. In three families—A17, AK, and AZ (fig. 1C)—marker genotypes were consistent with linkage to the DFNB28 region. No one in these three families carried the R347X mutation. However, sequence of the newly discovered exons in deaf individuals from these families revealed another nonsense mutation, 1741C→T (Q581X), in the same exon of TRIOBP (fig. 3B). All 12 deaf individuals in families A17, AK, and AZ were homozygous for Q581X. Both R347X and Q581X lead to truncation of the long TRIOBP isoform but are predicted to have no effect on the short TRIOBP isoform, since the latter is transcribed from an alternate first exon.
We screened R347X and Q581X in all deaf probands of the 156 families in our study. As mentioned above, families K, AX, AY, and BD, which harbor R347X, are Orthodox Christian. Families A17, AK, and AZ, which harbor Q581X, are Muslim. Because the Palestinian Orthodox Christian and Muslim communities are each highly endogamous, it is not surprising that they harbor different mutations, though in the same gene. It was interesting, therefore, to identify family X7, in which the two deaf children were both compound heterozygous for R347X and Q581X.
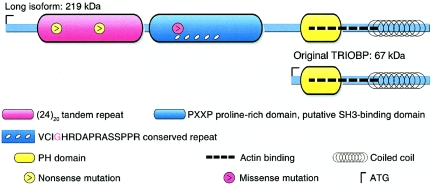
Among the probands genotyped for the two nonsense mutations was a child of age 3 years with profound hearing loss who was heterozygous for R347X and did not carry the Q581X mutation. The child inherited R347X from her hearing father. We fully sequenced the long isoform of TRIOBP in this child and found her to be heterozygous for another novel mutation, 3055G→A (G1019R), which was inherited from her hearing mother (fig. 3C). G1019R is a nonconservative amino acid substitution in a 521-aa proline-rich region (fig. 4). Analysis with bioinformatics tools suggests that this region may function as an SH3-binding domain (Ren et al. 1993; fig. 5); three PXXP-containing SH3-binding sites are predicted in this region by the iSPOT algorithm (Brannetti and Helmer-Citterich 2003). Ligand preferences at SH3-binding sites are determined by motifs that may lie some distance from a PXXP-containing peptide (Kuriyan and Cowburn 1997). G1019R lies in a repeated 16-aa motif that is highly conserved among mammals but does not appear in any proteins other than TRIOBP. We speculate that this motif may be involved in determining ligand preference at this site and that G1019R may disrupt this role. Future work will test this hypothesis.
Figure 4.
Proline-rich domain at aa 879–1399 of the long isoform of human TRIOBP that harbors the missense mutation G1019R (G in bold and inside a box) in a 16-aa conserved repeat (underlined). This region has properties of an SH3-binding domain (Ren et al. 1993); three PXXP-containing SH3-binding domains (in boxes) are predicted by the iSPOT algorithm (Brannetti and Helmer-Citterich 2003). Ligand preferences at SH3-binding sites are determined by arginine or threonine motifs at some distance from the PXXP peptides (Kuriyan and Cowburn 1997). We speculate that the conserved motifs may be involved in specifying ligand preference and that substitution of arginine for glycine may disrupt this role. This region is truncated by both nonsense mutations.
Figure 5.
Predicted functional domains of the short and long isoforms of TRIOBP
We screened the three TRIOBP mutations in 300 unrelated Palestinian hearing controls from different areas of the West Bank; one individual was heterozygous for G1019R, and none carried R247X or Q581X. On the basis of the families reported above, the frequencies of the three mutant alleles among the 156 probands with hereditary hearing loss were 0.029 for R247X, 0.032 for Q581X, and 0.003 for G1019R.
The known features of TRIOBP, combined with the restricted expression of the long isoform, suggest a role for the long isoform of TRIOBP in hearing loss. The short isoform of TRIOBP is a widely expressed 67-kDa protein with an N-terminal pleckstrin homology (PH) domain and a C-terminal coiled-coil region (fig. 5). Ectopically expressed TRIOBP localizes in a periodic fashion to F-actin and enhances cell spreading, and in vitro binding studies indicate direct interaction between TRIOBP and F-actin (Seipel et al. 2001). Furthermore, cells with stably or transiently overexpressed TRIOBP displayed extensively flattened morphology with enhanced stress fibers and cortical F-actin. TRIOBP-expressing cells were also resistant to the F-actin destabilizer latruniculin B, which indicates that TRIOBP stabilizes F-actin structures (Seipel et al. 2001). Unlike other actin-binding proteins, TRIOBP lacks conventional actin-binding domains (Sobue and Sellers 1991). The actin cytoskeleton is essential for different cellular functions, including cell shape and movement and membrane trafficking, with specialized actin architecture in the inner ear. The hearing process and auditory transduction depends on the development and proper function of hair cells in the cochlea that contain a group of projections of actin-filled stereocilia from their apical cell surfaces (Hudspeth 1997). These stereocilia develop from microvilli on the apical hair cell surface, in a process that involves elongation and broadening of actin filaments (Kaltenbach and Falzarano 1994; Kollmar 1999; Muller and Littlewood-Evans 2001).
Given the possible role of TRIOBP in actin cytoskeletal organization, we examined its localization in the inner ear—in particular, localization to the actin cytoskeleton. Cochlear sensory epithelia, including the organ of Corti, the stria vascularis, and the modiolus, were dissected from neonatal mice at postnatal days P0–P5. All procedures involving animals met the guidelines described in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committees of Tel Aviv University and the University of Washington. Samples were treated as described elsewhere (Rhodes et al. 2004).
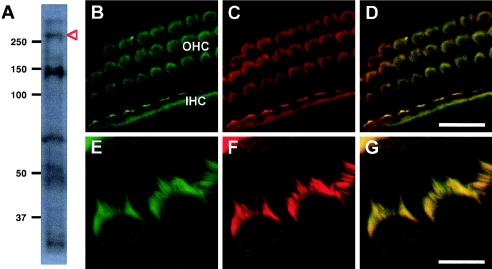
We performed western analysis of protein from mouse cochlea, using the monoclonal antibody TARA.27 (kindly provided by K. Seipel and M. Streuli), which was raised against aa 13–185 of original TRIOBP, equivalent to aa 1793–2165 of the long isoform. As expected (Seipel et al. 2001), we detected an isoform of ∼70 kDa (fig. 6A). We also detected isoforms of ∼150 kDa and >250 kDa. The predicted size of the long isoform of TRIOBP in mouse is 219 kDa. We speculate that the >250-kDa fragment may correspond to a posttranslationally modified product of the 219-kDa isoform and that the ∼150-kDa fragment may be an as-yet-uncharacterized breakdown product of this isoform or another alternatively spliced product.
Figure 6.
Expression and localization of TRIOBP in mouse cochlea. A, Western blot of two cochlea from newborn (P0) mice hybridized with monoclonal antibody TARA.27 (Seipel et al. 2001). Bands of ∼70 kDa and ∼50 kDa may represent two isoforms of original TRIOBP (UCSC Human Genome Browser May 2004 assembly). The band of >250 kDa (red arrow) may represent posttranslational modification of the predicted 219-kDa novel TRIOBP isoform. The band of ∼150 kDa may represent either a breakdown product of this isoform or an as-yet-uncharacterized alternatively spliced isoform. B–D, Top view of P0 whole-mount cochleae stained with monoclonal antibody TARA.27 against TRIOBP (B) and with rhodamine phalloidin, which stains F-actin (C). The protein is detected in the stereocilia of both the outer hair cells (OHC) and inner hair cells (IHC). Labeling indicates colocalization with actin along the length of the stereocilia and in the microvilli on the apical portion of the hair cells (D). E–G, Higher magnification showing stereocilia of outer hair cells. Scale bar is 16 μm in panels B–D and 4 μm in E–G.
The TARA.27 antibody revealed expression of TRIOBP in the sensory epithelium of the inner ear (fig. 6B–6G). TRIOBP is expressed in both the inner hair cells and outer hair cells of the organ of Corti. TRIOBP colocalizes with the phalloidin-stained F-actin along the length of the stereocilia and in the microvilli, which are actin-based projections that precede stereocilia and persist a few days after birth before being resorbed. Because the TARA.27 epitope is shared by the short and long isoforms of TRIOBP, we cannot distinguish relative amounts of the two isoforms. Localization of these proteins in the cochlear sensory hair cells is consistent with a role for TRIOBP in the actin cytoskeleton and with the genetic evidence indicating that the long isoform of TRIOBP is the gene responsible for DFNB28 hearing loss.
Acknowledgments
We thank the students, families, and staff of the Palestinian schools for children with hearing loss, for their enthusiastic participation. We thank Katja Seipel and Michel Streuli for TARA.27 and TARA.397 antibodies to TRIOBP, Dr. Wa’el Salhab for clinical examinations, and Amiel Dror for figures. H.S. is supported by the Canadian International Scientific Exchange Program (CISEPO). This project was supported by NIH grant R01 DC005641. This work was performed by H.S. in partial fulfillment of the requirements for a Ph.D. degree, Sackler Faculty of Medicine, Tel Aviv University.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for mouse long isoform of TRIOBP [accession number DQ278602], human long isoform of TRIOBP [accession number DQ278603], and transcripts from brain [accession number AB051449] and fetal brain [accession number AK096634])
- Hereditary Hearing Loss Homepage, http://webhost.ua.ac.be/hhh/
- UCSC Genome Browser, http://genome.ucsc.edu/
References
- Baldwin CT, Weiss S, Farrer LA, De Stefano AL, Adair R, Franklyn B, Kidd KK, Korostishevsky M, Bonne-Tamir B (1995) Linkage of congenital, recessive deafness (DFNB4) to chromosome 7q31 and evidence for genetic heterogeneity in the Middle Eastern Druze population. Hum Mol Genet 4:1637–1642 [DOI] [PubMed] [Google Scholar]
- Bonne-Tamir B, DeStefano AL, Briggs CE, Adair R, Franklyn B, Weiss S, Korostishevsky M, Frydman M, Baldwin CT, Farrer LA (1996) Linkage of congenital recessive deafness (DFNB10) to chromosome 21q22.3. Am J Hum Genet 58:1254–1259 [PMC free article] [PubMed] [Google Scholar]
- Brannetti B, Helmer-Citterich M (2003) iSPOT: a web tool to infer the interaction specificity of families of protein modules. Nucleic Acids Res 31:3709–3711 10.1093/nar/gkg592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DA, McHale DP, Brown KA, Moynihan LM, Houseman M, Karbani G, Parry G, Janjua AH, Newton V, al-Gazali L, Markham AF, Lench NJ, Mueller RF (1997) A new locus for non-syndromal, autosomal recessive, sensorineural hearing loss (DFNB16) maps to human chromosome 15q21-q22. J Med Genet 34:1015–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham RW Jr, Idury RM, Schaffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- Greinwald JH Jr, Wayne S, Chen AH, Scott DA, Zbar RI, Kraft ML, Prasad S, Ramesh A, Coucke P, Srisailapathy CR, Lovett M, Van Camp G, Smith RJ (1998) Localization of a novel gene for nonsyndromic hearing loss (DFNB17) to chromosome region 7q31. Am J Med Genet 78:107–113 [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ (1997) How hearing happens. Neuron 19:947–950 10.1016/S0896-6273(00)80385-2 [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Falzarano PR (1994) Postnatal development of the hamster cochlea. I. Growth of hair cells and the organ of Corti. J Comp Neurol 340:87–97 10.1002/cne.903400107 [DOI] [PubMed] [Google Scholar]
- Kollmar R (1999) Who does the hair cell’s “do”? Rho GTPases and hair-bundle morphogenesis. Curr Opin Neurobiol 9:394–398 10.1016/S0959-4388(99)80059-2 [DOI] [PubMed] [Google Scholar]
- Kuriyan J, Cowburn D (1997) Modular peptide recognition domains in eukaryotic signaling. Annu Rev Biophys Biomol Struct 26:259–288 10.1146/annurev.biophys.26.1.259 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML, Ploughman LM, Rawlings B, Remington E, Arnos KS, Nance WE (1993) Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am J Med Genet 46:486–491 10.1002/ajmg.1320460504 [DOI] [PubMed] [Google Scholar]
- Mburu P, Mustapha M, Varela A, Weil D, El-Amraoui A, Holme RH, Rump A, Hardisty RE, Blanchard S, Coimbra RS, Perfettini I, Parkinson N, Mallon AM, Glenister P, Rogers MJ, Paige AJ, Moir L, Clay J, Rosenthal A, Liu XZ, Blanco G, Steel KP, Petit C, Brown SD (2003) Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat Genet 34:421–428 10.1038/ng1208 [DOI] [PubMed] [Google Scholar]
- Medlej-Hashim M, Mustapha M, Chouery E, Weil D, Parronaud J, Salem N, Delague V, Loiselet J, Lathrop M, Petit C, Megarbane A (2002) Non-syndromic recessive deafness in Jordan: mapping of a new locus to chromosome 9q34.3 and prevalence of DFNB1 mutations. Eur J Hum Genet 10:391–394 10.1038/sj.ejhg.5200813 [DOI] [PubMed] [Google Scholar]
- Muller U, Littlewood-Evans A (2001) Mechanisms that regulate mechanosensory hair cell differentiation. Trends Cell Biol 11:334–342 10.1016/S0962-8924(01)02046-3 [DOI] [PubMed] [Google Scholar]
- Mustapha M, Chouery E, Chardenoux S, Naboulsi M, Paronnaud J, Lemainque A, Megarbane A, Loiselet J, Weil D, Lathrop M, Petit C (2002) DFNB31, a recessive form of sensorineural hearing loss, maps to chromosome 9q32-34. Eur J Hum Genet 10:210–212 10.1038/sj.ejhg.5200780 [DOI] [PubMed] [Google Scholar]
- Ren R, Mayer BJ, Cicchetti P, Baltimore D (1993) Identification of a ten-amino acid proline-rich SH3 binding site. Science 259:1157–1161 [DOI] [PubMed] [Google Scholar]
- Rhodes CR, Hertzano R, Fuchs H, Bell RE, de Angelis MH, Steel KP, Avraham KB (2004) A Myo7a mutation cosegregates with stereocilia defects and low-frequency hearing impairment. Mamm Genome 15:686–697 10.1007/s00335-004-2344-x [DOI] [PubMed] [Google Scholar]
- Scott HS, Kudoh J, Wattenhofer M, Shibuya K, Berry A, Chrast R, Guipponi M, Wang J, Kawasaki K, Asakawa S, Minoshima S, Younus F, Mehdi SQ, Radhakrishna U, Papasavvas MP, Gehrig C, Rossier C, Korostishevsky M, Gal A, Shimizu N, Bonne-Tamir B, Antonarakis SE (2001) Insertion of β-satellite repeats identifies a transmembrane protease causing both congenital and childhood onset autosomal recessive deafness. Nat Genet 27:59–63 [DOI] [PubMed] [Google Scholar]
- Seipel K, O’Brien SP, Iannotti E, Medley QG, Streuli M (2001) TARA, a novel F-actin binding protein, associates with the Trio guanine nucleotide exchange factor and regulates actin cytoskeletal organization. J Cell Sci 114:389–399 [DOI] [PubMed] [Google Scholar]
- Sobue K, Sellers JR (1991) Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem 266:12115–12118 [PubMed] [Google Scholar]
- Verpy E, Masmoudi S, Zwaenepoel I, Leibovici M, Hutchin TP, Del Castillo I, Nouaille S, Blanchard S, Laine S, Popot JL, Moreno F, Mueller RF, Petit C (2001) Mutations in a new gene encoding a protein of the hair bundle cause non-syndromic deafness at the DFNB16 locus. Nat Genet 29:345–349 10.1038/ng726 [DOI] [PubMed] [Google Scholar]
- Walsh TD, Shahin H, Morrow J, King M-C, Lynch E, Avraham KB, Kanaan M (2000) DFNB28, a novel locus for prelingual nonsyndromic autosomal recessive hearing loss maps to 22q13 in a large consanguineous Palestinian kindred. Poster presented at 50th Annual Meeting of American Society of Human Genetics, Philadelphia, October 3–7 [Google Scholar]
- Zwaenepoel I, Mustapha M, Leibovici M, Verpy E, Goodyear R, Liu XZ, Nouaille S, Nance WE, Kanaan M, Avraham KB, Tekaia F, Loiselet J, Lathrop M, Richardson G, Petit C (2002) Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc Natl Acad Sci USA 99:6240–6245 10.1073/pnas.082515999 [DOI] [PMC free article] [PubMed] [Google Scholar]