To the Editor:
In the October 2005 issue of the Journal, Benito-Sanz et al. (2005) reported an association of Léri-Weill dyschondrosteosis (LWD [MIM 127300]) with a novel class of heterozygous pseudoautosomal region 1 (PAR1) deletions downstream of SHOX (short-stature homeobox-containing gene [MIM 312865]) in 12 patients with two copies of intact SHOX coding sequences. The deletions were variable in size, with the smallest region of overlapping deletion (SRO) of ∼30 kb between DXYS10086 and rs7067102. The results—in conjunction with the report of Flanagan et al. (2002) describing a monoallelic SHOX expression in the bone marrow fibroblasts taken from the distal radius of a patient with LWD with two copies of normal SHOX coding exons and hemizygosity for a region around DXYS233 downstream of SHOX—suggest the presence of a downstream enhancer for SHOX transcription around the ∼30-kb SRO. Consistent with this, Fukami et al. (2005) found that (1) a 240–350-kb deletion including DXYS233 is present in a heterozygous status in a mother with LWD and two copies of intact SHOX coding exons and (2) the same deletion is present in a hemizygous status in her daughter with Langer mesomelic dysplasia and a mosaic-ring X chromosome missing the PAR1. Here, we report that the putative SHOX enhancer may reside on an ∼800-bp evolutionarily conserved sequence (ECS).
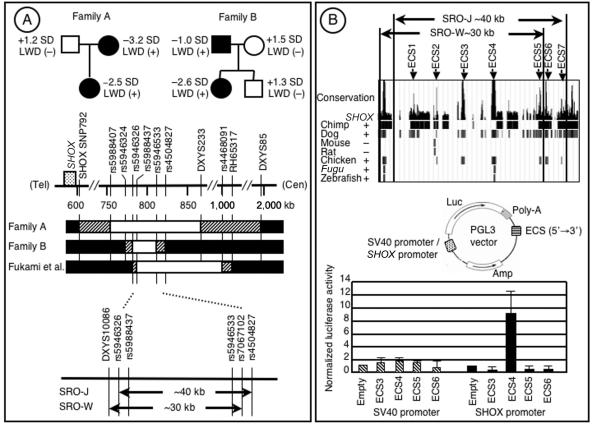
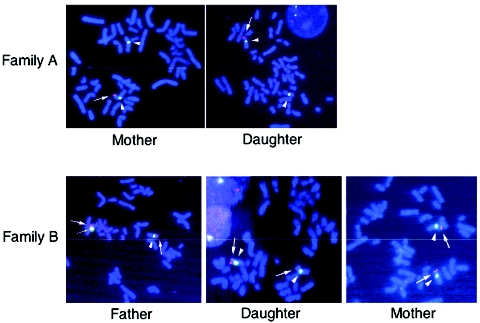
First, we analyzed the SHOX 3′ region in five Japanese families in which the proband and one of the parents had variable degrees of LWD and stature from short to low normal in the presence of two copies of intact SHOX-coding exons. The study was approved by the Institutional Review Board Committee at the National Center for Child Health and Development. Genotyping analysis was performed with primers and methods shown in table 1; results indicate that a deletion between SHOX-SNP792 on the 3′ UTR and DXYS85 was shared by the LWD-affected mother and daughter in family A and that a deletion between rs5946324 and rs4504827 was common to the LWD-affected father and daughter in family B (table 2 and fig. 1A). Furthermore, FISH analysis was performed with an RP13-167H21 BAC probe defining a region from rs5946324 to DXYS233 (Ensembl Genome Browser); results show only a single signal in the mother and the daughter of family A and an obviously different signal intensity in the father and the daughter of family B (fig. 2). The results, together with our previous data (Fukami et al. 2005), imply that an ∼40-kb region between rs5946326 and rs4504827 is the SRO in the Japanese patients (SRO-J) (fig. 1A). The SRO-J is in a close agreement with the ∼30-kb SRO in the white patients (SRO-W) (Benito-Sanz et al. 2005), and a region between rs5946326 and rs7067102 is shared by all the patients with SHOX 3′ deletions (fig. 1A).
Table 1.
Primer Sequences and PCR Conditions Used for Genotyping[Note]
|
Primer(5′→3′) |
||||
| Loci | Forward | Reverse | ATa(°C) | Size(bp) |
| SHOX (CA)n | CATGTCATATATATATGTGATCC | GACACAGAAATCCTTCATAAA | 55 | 141–155 |
| SHOXa/b +3/1239 and SHOXa/b +3/1248b | TTTAACCGAAATGAAGCCGAGA | AAATGTGTGTCGTGGGAGTGG | 57 | 230 |
| SHOX-SNP657 and SHOX-SNP792b | ATTGATGGTTAGTATTTTTTGTAGCAGTTG | TTAAAAATAAAGTTACAAAGGCCGGG | 65 | 681 |
| rs5988407 | TGGCAAATGGATCACTTCAA | CTTGGGGTTTCCCAGAAAAA | 56 | 376 |
| rs7055778 | AGGAGTGAGTATATCAAGACTTGGG | AGCTCTGCCTTGAGGTTGGA | 58 | 99 |
| rs5946324 | AAAATCACCGTCGTTTGGGAG | AGATCATTTACAGCAGAAGCCG | 58 | 195 |
| rs5946325 | AGGTGATTAGGGAAGCTCCGTGTT | TCGCTGTGCTGGAAGCAGAA | 60 | 162 |
| rs5946326 | ACGTTAAGGGAAATGAGCCA | TTTGTCTCTTTTCAGGACTAAGGA | 56 | 110 |
| rs5988281, rs5946329, rs5988432, and rs4946331c | GAAACAGGACAATACCAGTGCAGT | AATCACACTGATGTGAAATTGGG | 60 | 576 |
| rs5988437 | TGTTCTGTAAGTTGCATGTCTGCT | TGCTTTTCCTGGGTAGAAGGTA | 58 | 224 |
| rs6644384 | AAAGAAACAAGAGCTGCGTTCC | TCTATACGGGGGCTTGAAATGT | 56 | 280 |
| rs5946336 | CTTATTTGCCCCATCTCAGC | AAAAAGAGGAATTGTCATTCTATGT | 60 | 103 |
| rs7067102 and rs5946533b | TTTGCATCAGGAATCCATTTG | CCAAAGTAATGCGTAGACTTTG | 60 | 384 |
| rs4504827 | GAAATGCAAAATAGTGGCCGAG | CTTTTACGCATTGGATCCAGTG | 60 | 276 |
| rs5988299, rs5988300, rs5988301, and rs5988494c | AGGATCCCAGACGCCACTCAA | GCATTCATCTGCTGCAGGAATT | 58 | 261 |
| DXYS233 | TGGGAATTCGAGGCTG | TGATTTCCATCCTGGGGT | 54 | 271–299 |
| rs4468091 | TCTCAAACTCAGGGTTGTTTCG | AATTCCTACAGCTATAGGTCCCAG | 62 | 337 |
| rs5946712 | GGAATTATAGGCTGAGATCCACAG | ACAACCCTACAAATTCTAGCCCTC | 58 | 223 |
| DXYS85 | TTTGCTGAGCACCTAGAAGG | TAGGTCCTCTAGGTGCAGGA | 60 | 78/82 |
Note.— SHOX (CA)n, DXYS233, and DXYS85 have been genotyped by determining the PCR product sizes on an ABI PRISM 310 autosequencer with GeneScan software (Applied Biosystems). Other SNPs have been genotyped by direct sequencing of the PCR products on a CEQ 8000 autosequencer (Beckman Coulter).
AT = annealing temperature.
Two polymorphisms are present in the PCR products.
Four polymorphisms are present in the PCR products.
Table 2.
Summary of Polymorphism Analyses[Note]
|
Allele or Polymorphism Position |
||||||
| Family A |
Family B |
|||||
| Locusa | Polymorphismb | DaughterLWD (+) | MotherLWD (+) | DaughterLWD (+) | FatherLWD (+) | MotherLWD (−) |
| SHOX (CA)nc | MS (CA)n | 141/153 |
141/149 |
149 | 149/153 |
149/153 |
| SHOXa/b +3/1239d | SNP (C/G) | C | C | G | C/G |
NE |
| SHOXa/b +3/1248d | SNP (G/A) | G | G | A | A/G |
NE |
| SHOX-SNP657e | SNP (G/A) | A | A/G |
NE | NE | NE |
| SHOX-SNP792f | SNP (T/G) | T/G |
T | T | T | T |
| rs5988407 | SNP (C/T) | C | T | NE | NE | NE |
| rs7055778 | SNP (G/A) | A | A | NE | NE | NE |
| rs5946324g | SNP (C/G) | G | G | G | G/C |
G/C |
| rs5946325g | SNP (C/T) | T | T | T | T | NE |
| rs5946326g | SNP (G/A) | G | G | A | G | NE |
| rs5988281g | SNP (C/G) | C | C | G | C | NE |
| rs5946329g | SNP (C/T) | C | C | T | C | NE |
| rs5988432g | SNP (C/T) | C | C | C | C | NE |
| rs5946331g | SNP (G/A) | G | G | A | G | NE |
| rs5988437g | SNP (C/G) | C | G | G | C | NE |
| rs6644384g | SNP (G/A) | A | A | NE | NE | NE |
| rs5946336g | SNP (G/A) | A | A | G | A | NE |
| rs7067102g | SNP (G/A) | NE | NE | A | A | NE |
| rs5946533g | SNP (C/T) | NE | NE | T | C | NE |
| rs4504827g | SNP (T/A) | T | T | T/A |
T/A |
NE |
| rs5988299g | SNP (C/T) | NE | NE | C | C | NE |
| rs5988300g | SNP (G/A) | NE | NE | G | A/G |
NE |
| rs5988301g | SNP (C/G) | NE | NE | G | G/C |
NE |
| rs5988494g | SNP (C/G) | NE | NE | C | C | NE |
| DXYS233g | MS (CA)n | 273 | 279 | 273 | 273/283 |
273/279 |
| rs4468091 | SNP (G/A) | G | G | G | G | A/G |
| rs5946712 | SNP (G/A) | G | G | G | G | G |
| DXYS85 | 4-bp ins/del | 82 | 78/82 |
78/82 |
82 | 78/82 |
Note.— The loci present in two copies are underlined, and those not shared by the LWD-affected proband and parent are in bold italics. A plus sign (+) = affected; a minus sign (−) = not affected; NE = not examined.
SHOXa/b +3/1239 and SHOXa/b +3/1248 are based on the Polymorphisms around SHOX Database, SHOX-SNP657 and SHOX-SNP792 are based on the study by Flanagan et al. (2002), and the remaining loci are based on dbSNP and dbSTS databases.
MS = microsatellite.
Located in the SHOX 5′ UTR.
Located between exon 6a inherent to SHOXa and exon 6b specific to SHOXb.
Silent polymorphism on exon 6b.
Located in the SHOX 3′ UTR.
Loci included in the RP13-167H21 BAC probe used for FISH analysis.
Figure 1.
A, PAR1 deletions in the SHOX downstream region. Top, Pedigrees of families A and B. LWD is exhibited by the mother and the daughter in family A and by the father and the daughter in family B. The height of each subject is expressed as an SD score. Bottom, Deletion maps of the SHOX 3′ region. In families A and B, the blackened segments represent the disomic regions, the unblackened segments depict the monosomic regions, and the striped segments indicate the dosage-unknown region in which the breakpoints should exist. The physical distance (kb) from the Xp/Yp telomere (“Tel”) is shown below the horizontal line. The results of the present study and those reported by Fukami et al. (2005) indicate that the SRO-J spans ∼40 kb in physical length and is largely similar to the SRO-W reported by Benito-Sanz et al. (2005). B, Functional analysis of the ECSs. Top, Seven ECSs (ECS1–ECS7) are identified in the SRO-J (UCSC Genome Browser). Bottom, Transcription analysis of ECS3–ECS6. A luciferase reporter construct has been created with the SV40 or the human SHOX promoter, and each ECS inserted into the 3′ region of the luciferase gene (“Luc”). Only the combination of the SHOX promoter and ECS4 has significantly increased the luciferase activity.
Figure 2.
Results of the FISH analysis. Red signals indicate the RP13-167H21 region (thick arrows), and green signals represent DXZ1 (arrowheads) or DYZ3 (thin arrow). The RP13-167H21 region is deleted from one of the two X chromosomes of the mother and the daughter in family A. The signal for RP13-167H21 is obviously weak in one of the sex chromosomes of the father and the daughter in family B, whereas it is similar between the two X chromosomes of the mother.
Next, we searched the UCSC Genome Browser for the ECSs within the SROs. Seven ECSs (ECS1–ECS7) were present within the SRO-J, whereas ECS6 and ECS7 were found to reside outside the SRO-W (fig. 1B). ECS3–ECS7 were well conserved in chicken and dog, which preserve Shox, and were absent in mouse and rat, which lack Shox (Clement-Jones et al. 2000; Ensembl Genome Browser). ECS4 was also conserved in Fugu and zebrafish, which preserve Shox. By contrast, ECS1 was absent in chicken, and ECS2 was conserved in mouse and rat. Whereas ECS3 and ECS4 were not described in chimpanzee, the sequence analysis remains poor for the Shox 3′ region in chimpanzee, in contrast to the detailed analysis of that region in chicken and dog. These findings suggest that ECS3–ECS5 can be regarded as candidate regions harboring the putative downstream enhancer. In this regard, since ECS3–ECS5 reside between rs5988437 and rs5946533 (UCSC Genome Browser), they should be deleted from the patients in the three families defining the SRO-J (fig. 1A).
Thus, we examined the transcription activity of ECS3–ECS5 as well as ECS6 with the dual-luciferase reporter assay system (Promega). Luciferase reporter constructs containing each ECS (ECS3, 861 bp; ECS4, 824 bp; ECS5, 441 bp; ECS6, 634 bp) inserted into the 3′ region of the luciferase gene were created using the pGL3 vector with the SV40 promoter or the human SHOX promoter on exon 2 (−432 to +5 bp) (Blaschke et al. 2003) (fig. 1B). The U2OS osteosarcoma cell line expressing SHOX (Rao et al. 2001) was transfected using lipofectamine (Invitrogen) with each reporter vector together with the pRL-CMV vector used as an internal control for the transfection, and luciferase assays were performed 36 h later. After the experiments were performed five times, the normalized luciferase activity was found to be significantly increased only when the reporter vector with ECS4 and the SHOX promoter was transfected to U2OS cells (empty vs. ECS4; P=.011 by t test) (fig. 1B). This implies that the putative SHOX enhancer resides in ECS4 and interacts with the SHOX promoter on exon 2.
Finally, we searched ECS4 for potential binding sites for transcription factors relevant to bone development, using the MATINSPECTOR, TESS, and TFSEARCH programs. The putative binding sites with the maximum core similarity of 1.0 and a matrix similarity >0.75 were identified for HOXA9, HOXB9, PBX1, and MEIS1, as well as for PBX1-HOXA9 and MEIS1-HOXA9 heterodimers, which are known to be involved in limb development (Mercader et al. 1999; Shanmugam et al. 1999; Zákány and Duboule 1999) (fig. 3). Thus, the sequence-specific DNA binding of such a factor(s) might mediate the enhancer activity for SHOX. However, other potential binding sites were also detected for various transcription factors, and the relevance to skeletal development has not been studied or excluded in most of the transcription factors. In addition, the binding sites remain unidentified for many transcription factors. Thus, further studies are necessary to define the enhancer sequence.
Figure 3.
Nucleotide sequence of ECS4 and putative binding sites for several transcription factors relevant to skeletal development. The human sequence is aligned with the chicken sequence.
In summary, the results suggest that the ∼800-bp ECS4 harbors the putative downstream enhancer for SHOX transcription. This information will provide a useful clue for the clarification of the molecular network involved in SHOX-dependent skeletal development.
Acknowledgments
This work was supported in part by Ministry of Health, Labor and Welfare grant 17-C2 for child health and development and by grant-in-aid 17591132 from the Ministry of Education, Science, Sports, and Culture.
Web Resources
The URLs for data presented herein are as follows:
- dbSNP, http://www.ncbi.nlm.nih.gov/projects/SNP/
- dbSTS, http://www.ncbi.nlm.nih.gov/dbSTS/
- Ensembl Genome Browser, http://www.ensembl.org/
- MATINSPECTOR, http://www.genomatix.de/products/MatInspector/MatInspector3.html
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for LWD and SHOX)
- Polymorphisms around SHOX Database, http://www.le.ac.uk/genetics/ajj/SHOX/mapdetails.html
- TESS: Transcription Element Search System, http://www.cbil.upenn.edu/tess/
- TFSEARCH, http://www.cbrc.jp/research/db/TFSEARCH.html
- UCSC Genome Browser, http://genome.ucsc.edu/
References
- Benito-Sanz S, Thomas NS, Huber C, Del Blanco DG, Aza-Carmona M, Crolla JA, Maloney V, Argente J, Campos-Barros Á, Cormier-Daire V, Heath KE (2005) A novel class of pseudoautosomal region 1 deletions downstream of SHOX is associated with Léri-Weill dyschondrosteosis. Am J Hum Genet 77:533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke RJ, Topfer C, Marchini A, Steinbeisser H, Janssen JW, Rappold GA (2003) Transcriptional and translational regulation of the Leri-Weill and Turner syndrome homeobox gene SHOX. J Biol Chem 278:47820–47826 10.1074/jbc.M306685200 [DOI] [PubMed] [Google Scholar]
- Clement-Jones M, Schiller S, Rao E, Blaschke RJ, Zuniga A, Zeller R, Robson SC, Binder G, Glass I, Strachan T, Lindsay S, Rappold GA (2000) The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum Mol Genet 9:695–702 10.1093/hmg/9.5.695 [DOI] [PubMed] [Google Scholar]
- Flanagan SF, Munns CF, Hayes M, Williams B, Berry M, Vickers D, Rao E, Rappold GA, Batch JA, Hyland VJ, Glass IA (2002) Prevalence of mutations in the short stature homeobox containing gene (SHOX) in Madelung deformity of childhood. J Med Genet 39:758–763 10.1136/jmg.39.10.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami M, Okuyama T, Yamamori S, Nishimura G, Ogata T (2005) Microdeletion in the SHOX 3′ region associated with skeletal phenotypes of Langer mesomelic dysplasia in a 45,X/46,X,r(X) infant and Leri-Weill dyschondrosteosis in her 46,XX mother: implication for the SHOX enhancer. Am J Med Genet A 137:72–76 [DOI] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Azpiazu N, Serrano A, Morata G, Martinez C, Torres M (1999) Conserved regulation of proximodistal limb axis development by Meis1/Hth. Nature 402:425–429 10.1038/46580 [DOI] [PubMed] [Google Scholar]
- Rao E, Blaschke RJ, Marchini A, Niesler B, Burnett M, Rappold GA (2001) The Leri-Weill and Turner syndrome homeobox gene SHOX encodes a cell-type specific transcriptional activator. Hum Mol Genet 10:3083–3091 10.1093/hmg/10.26.3083 [DOI] [PubMed] [Google Scholar]
- Shanmugam K, Green NC, Rambaldi I, Saragovi HU, Featherstone MS (1999) PBX and MEIS as non-DNA-binding partners in trimeric complexes with HOX proteins. Mol Cell Biol 19:7577–7588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zákány J, Duboule D (1999) Hox genes in digit development and evolution. Cell Tissue Res 296:19–25 10.1007/s004410051262 [DOI] [PubMed] [Google Scholar]