Abstract
Ubiquinone (coenzyme Q10 or CoQ10) is a lipid-soluble component of virtually all cell membranes, where it functions as a mobile electron and proton carrier. CoQ10 deficiency is inherited as an autosomal recessive trait and has been associated with three main clinical phenotypes: a predominantly myopathic form with central nervous system involvement, an infantile encephalomyopathy with renal dysfunction, and an ataxic form with cerebellar atrophy. In two siblings of consanguineous parents with the infantile form of CoQ10 deficiency, we identified a homozygous missense mutation in the COQ2 gene, which encodes para-hydroxybenzoate-polyprenyl transferase. The A→G transition at nucleotide 890 changes a highly conserved tyrosine to cysteine at amino acid 297 within a predicted transmembrane domain. Radioisotope assays confirmed a severe defect of CoQ10 biosynthesis in the fibroblasts of one patient. This mutation in COQ2 is the first molecular cause of primary CoQ10 deficiency.
In the mitochondrial respiratory chain, coenzyme Q10 (CoQ10) (MIM 607426) is vital for the transport of electrons from complex I (NADH-ubiquinone oxidoreductase) and complex II (succinate-ubiquinone oxidoreductase) to complex III (ubiquinol-cytochrome c reductase) (Turunen et al. 2004). It is also an antioxidant (Kagan et al. 1990) and a membrane stabilizer (Turunen et al. 2004), and its oxidized form serves as a cofactor for uncoupling proteins in brown adipose tissue (Echtay et al. 2000). Increased levels of CoQ10 may protect cells from chemotherapy-induced oxidative stress (Brea-Calvo et al., in press).
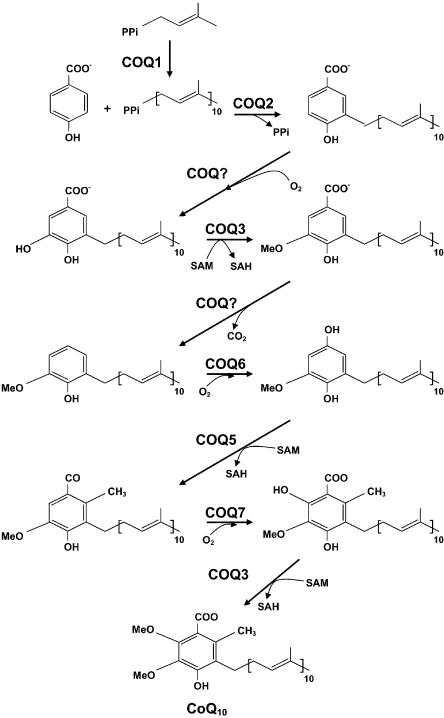
Deficiency of CoQ10 has been associated with three major clinical phenotypes. A predominantly myopathic form is characterized by recurrent myoglobinuria and CNS involvement with seizures, ataxia, or mental retardation (Ogasahara et al. 1989; Sobreira et al. 1997; Boitier et al. 1998; Lalani et al. 2005). A second variant, described in three families, manifests as an infantile encephalomyopathy with renal involvement (Rötig et al. 2000; Rahman et al. 2001; Salviati et al. 2005). The third variant is dominated clinically by ataxia and cerebellar atrophy, with varying involvement of other regions of the CNS, peripheral nerve, and muscle (Musumeci et al. 2001; Lamperti et al. 2003). In three siblings with cerebellar ataxia and CoQ10 deficiency, we identified a homozygous stop codon mutation in the APTX gene (Quinzii et al. 2005), which is known to cause ataxia and oculomotor apraxia 1 (AOA1) (Date et al. 2001; Moreira et al. 2001). This finding supports the hypothesis that the ataxic form is a genetically heterogeneous entity in which deficiency of CoQ10 can be secondary. Patients with all three forms of CoQ10 deficiency have shown clinical improvements after initiating oral CoQ10 supplementation. Thus, early diagnosis is of critical importance in the management of these patients. The molecular bases for the CoQ10 deficiency in most of these patients remain to be identified and presumably involve defects of CoQ10 biosynthesis (fig. 1).
Figure 1.
CoQ10 biosynthetic pathway with eight known biosynthetic enzymes denoted as COQ1–COQ8. COQ2, decaprenyl-4-hydroxybenzoate transferase, mediates the conjugation of the benzoquinone ring with the decaprenyl side chain.
We reported a 33-mo-old boy with infantile encephalomyopathy, nephropathy, and deficiency of CoQ10 (Salviati et al. 2005). The disease appeared to be an autosomal recessive trait because the patient’s parents were first cousins and his 9-mo-old sister with nephropathy also had CoQ10 deficiency in fibroblasts. The proband presented with proteinuria at age 12 mo; a renal biopsy revealed focal and segmental glomerulosclerosis. Neurological evaluation showed hypotonia, mild psychomotor delay, and optic atrophy. His renal function worsened. At age 18 mo, he developed frequent vomiting, and peritoneal dialysis was initiated. He developed psychomotor regression (loss of the ability to walk or stand unassisted), tremor, and new-onset status epilepticus with focal electroencephalogram abnormalities predominantly in the left occipital region. Brain magnetic resonance imaging showed cerebellar atrophy, mild diffuse cerebral atrophy, and stroke-like lesions in the left cingulate cortex and subcortical area. Blood and cerebrospinal fluid lactate levels were normal. At age 22 months, he developed right hemiplegia, myoclonus, and swallowing difficulties. A muscle biopsy revealed myofibers with excessive succinate dehydrogenase staining but no ragged-red fibers or cytochrome c oxidase–deficient fibers. Measurement of respiratory chain enzymes in muscle extracts showed decreased activities of complexes I + III (0.38 μmol/min/g fresh tissue; control mean ± SD = 1.02 ± 0.38) and II + III (0.22 μmol/min/g; control mean ± SD = 0.70 ± 0.23), whereas other complexes had normal activities. CoQ10 concentration in skeletal muscle of the proband was 12 μg/g fresh tissue (mean ± SD of 185 controls = 32.1 ± 6.7). In fibroblasts, CoQ10 levels were more severely reduced in both patients (proband = 19 ng/mg protein; sister = 18 ng/mg; mean ± SD of 15 controls = 105 ± 14). Activities of complexes II and III in the fibroblasts of both patients were decreased (23% and 22%, respectively, of controls), but the defect was corrected after the addition of 50 μM decylubiquinone. After the initiation of CoQ10 supplementation, the neurological manifestations of the boy improved dramatically.
We performed homozygosity mapping, using fluorescently labeled microsatellite markers (ABI Prism Linkage Mapping Set MD-10 [Applied Biosystems]) of chromosomal loci for the eight known human genes (COQ1–COQ8) encoding CoQ10 biosynthetic proteins. Three chromosomal loci revealed homozygosity in the affected individuals: chromosomes 14q24 (COQ6), 12q24 (COQ5), and 4q21 (COQ2). Primer sequences and PCR conditions for amplification of candidate genes are available from the author on request. Sequencing of the three candidate genes in the patients revealed only one nonsynonymous change: in COQ2, encoding para-hydroxybenzoate (PHB)–polyprenyl transferase (EC 2.5.1.39), we found a homozygous A→G transition at nucleotide 890, which is predicted to change amino acid 297 from tyrosine to cysteine in the third of six predicted transmembrane domains of the COQ2 protein (Forsgren et al. 2004) (fig. 2). The transition was heterozygous in both parents and absent in DNA from 100 healthy individuals tested by RFLP analysis using AflII restriction endonuclease digestion of PCR-amplified DNA fragments. We also sequenced COQ1, to exclude a defect in transprenyl transferase. We did not identify any mutations in COQ2 by sequencing genomic DNA in seven additional patients with CoQ10 deficiency in skeletal muscle (three with the predominantly myopathic form, two with severe ataxia, and two with infantile-onset encephalomyopathy).
Figure 2.
Evolutionary conservation of COQ2. Human amino acid 297 is normally tyrosine (Y). The A→G transition at nucleotide 890 changes amino acid 297 to cysteine.
To confirm that the patients had a defect of CoQ10 biosynthesis, we performed biochemical assays to measure incorporation of each of two radiolabeled substrates (fig. 1). For the first assay, fibroblasts from the proband and a control were plated in six separate wells of 24×6.5–mm plates (40,000 cells/well) and were cultured using Dulbecco's modified Eagle medium (DMEM) with 20% fetal calf serum (Nishigaki et al. 2003). After four days, the medium was replaced by fresh DMEM with 20% fetal calf serum and 0.1 μCi of 14C-PHB (50 Ci/mol specific activity). After incubation for an additional 24 h, cells were washed twice with PBS and were detached from the bottom of the wells with the use of 0.5 ml of 1% SDS, followed by shaking for 10 min at room temperature (repeated once). The contents of each plate were combined (3 ml total), and 0.2 ml was saved for protein determination (Lowry et al. 1951). The remaining pooled suspensions were subjected to hexane extraction. CoQ10 was extracted after the addition of 4 ml hexane-ethanol (5/2 v/v) and was vortexed for 2 min. After centrifugation at 2,500 rpm at room temperature for 5 min, the upper phase was carefully transferred into a 20-ml glass scintillation vial (performed twice for each sample). The combined extract was evaporated under a gentle stream of N2 gas, and the residue was dissolved in 0.15 ml of 1-propanol. An aliquot of 50 μl of the extract was directly injected into a high-performance liquid chromotography (HPLC) system with a C18 reversed-phase column and an electrochemical detector. The waste line of HPLC was connected to a fraction collector, which was programmed to collect 1.0 ml of fractions per minute. CoQ10 peak was identified by specific retention time determined after injection of a known amount of authentic CoQ10. The amount of radioactivity in the collected fraction was determined in a Packard scintillation counter.
In the second COQ2 assay, enzymatic activity in fibroblast lysate was measured by a described method (Kalen et al. 1990), with modifications. The incubation mixture contained 250 nCi of 3H-radiolabeled decaprenyl pyrophosphate (decaprenyl-PP) (20 Ci/mmol) solubilized in 25 μL of 1% Triton X-100, 50 mM potassium phosphate at pH 7.5, 10 mM MgCl2, 5 mM ATP, 20 μM 4-hydroxybenzoic acid, and fibroblast lysate (∼1 mg protein) in a total volume of 0.5 ml. After incubation at 37°C in a shaking water bath for 60 min, the reaction was stopped by the addition of 1 ml ethanol followed by 1 ml of 0.1-M SDS. Hexane extraction was performed as described above, and the residue was dissolved in 0.15 ml of 1-propanol. An aliquot of 50 μl was injected onto the HPLC. Fraction collection and radioactivity measurement were performed as described above.
In the first assay, after incubating fibroblasts from a control and patient 1 with 14C-PHB, the level of radiolabeled CoQ10 in the patient’s fibroblasts was ∼22% of the control mean (patient = 338 decays per min/mg protein/h; control mean ± SD = 1,733 ± 747, n=5) (table 1). In the second assay, homogenates from patient 1 and control fibroblasts incubated with 3H-radiolabeled decaprenyl-PP revealed that COQ2 activity in the patient was only 36% relative to the control mean (patient = 48 pmol/mg protein/h; control mean ± SD = 130 ± 18, n=5) (table 1). Unfortunately, the fibroblasts of the proband’s sister did not replicate sufficiently to allow measurement of CoQ10 biosynthesis in her cells.
Table 1.
Biochemical Assays to Measure COQ2 Activity
| Fibroblasts | CoQ10 Synthesisa | Percent ofControl Mean |
| Assay 1b: | ||
| Patient 1 | 388 DPM/mg protein/h | 22 |
| Control | 1,733 ± 747 DPM/mg protein/h | 100 |
| Assay 2c: | ||
| Patient 1 | 48 pmol/mg protein/h | 36 |
| Control | 130 ± 18 pmol/mg protein/h | 100 |
In both assays, radiolabeled CoQ10 was isolated by HPLC and was quantitated in a scintillation counter. Controls are measured as means ± SDs (n=5). DPM = decays per minute.
Cultured cells were incubated for 24 h with 0.1 μCi 14C-PHB (50 Ci/mol specific activity).
Fibroblast homogenates were incubated with 3H-radiolabeled decaprenyl-PP.
Cells synthesize CoQ10 de novo, starting with synthesis of the PHB ring and the polyisoprenyl tail, which anchors CoQ10 to membranes. The length of this tail varies among different organisms. In humans, the side chain is comprised of 10 isoprenyls producing CoQ10, whereas rats predominantly generate CoQ9. In yeast, mutations in any of the eight COQ genes block CoQ10 synthesis, and CoQ10-deficient cells cannot grow on nonfermentable carbon sources because of respiratory chain dysfunction, despite the accumulation of the intermediate demethoxy-CoQ10 (Gin et al. 2003). These yeast strains are also more sensitive to oxidative stress. These results indicate that CoQ10 cannot be replaced by another molecule in the cell and suggest that human diseases due to primary CoQ10 deficiency may present as severe mitochondrial encephalomyopathies.
Although deficiency of CoQ10 in skeletal muscle was originally described in 1989 and has been reported in at least 35 patients, the pathogenic mechanisms had been undefined (Ogasahara et al. 1989; Sobreira et al. 1997; Boitier et al. 1998; Rötig et al. 2000; Di Giovanni et al. 2001; Musumeci et al. 2001; Rahman et al. 2001; Van Maldergem et al. 2002; Lamperti et al. 2003; Aure et al. 2004; Gironi et al. 2004; Lalani et al. 2005). The wide variety of clinical presentations associated with CoQ10 deficiency has suggested genetic heterogeneity, probably related to the many steps involved in the CoQ10 biosynthesis.
We have identified the first molecular cause of primary CoQ10 deficiency, a mutation in the gene encoding PHB-polyprenyl transferase (COQ2), the second enzyme in the biosynthetic pathway of CoQ10 (fig. 1). PHB-polyprenyl transferase mediates the conjugation of the benzoquinone ring with the decaprenyl side chain and, thus, plays a central role in the biosynthesis of CoQ10 (Forsgren et al. 2004).
The G→A mutation at nucleotide 890 of COQ2 appears to exert a pathogenic effect by blocking ubiquinone synthesis. Evidence supporting pathogenicity includes: (1) the substitution of a well-conserved aromatic tyrosine amino acid by a polar uncharged cysteine in a predicted transmembrane domain of the protein, (2) absence of the mutation in 100 unrelated controls (200 alleles), and (3) the significantly decreased rate of CoQ10 synthesis, indicated by the reduced incorporation of radiolabeled PHB and decaprenyl-PP into CoQ10 in skin fibroblasts of the proband (23%–25% of control fibroblasts).
The pathogenic effects of CoQ10 deficiency in humans are uncertain. Lack of CoQ10 in mitochondria will disrupt the flow of reducing equivalents to respiratory chain complex III from complexes I and II, which, in turn, will lead to decreased ATP synthesis by oxidative phosphorylation. Respiratory chain defects typically cause encephalomyopathies due to the high energy requirements of these organs (DiMauro and Schon 2003); it is, therefore, not surprising that CoQ10 deficiency should also affect brain and muscle. In addition, because CoQ10 functions as an antioxidant, CoQ10 deficiency may preferentially affect postmitotic cells, such as neurons and muscle, which are particularly vulnerable to oxidative damage because they cannot replace dysfunctional cells. Furthermore, to function as an antioxidant, CoQ10 must be in the reduced form, but only 20% of the molecule is reduced in the brain (Naini et al. 2003). Brain vulnerability to oxidative stress has been demonstrated in neurodegenerative diseases, such as Friedreich ataxia (Puccio and Koenig 2002) and ataxia associated with deficiency of vitamin E (Yokota et al. 2001), which, like CoQ10, is a lipid-soluble antioxidant in biological membranes. Finally, in Schizosaccharomyces pombe with defective PHB-polyprenyltransferase, there is also increased vulnerability to oxidative stress, which is demonstrated both by increased sensitivity to pro-oxidants like H2O2 and Cu2+ and by the ability of supplemental antioxidants (cysteine, glutathione, and α-tocopherol) to restore growth in glucose medium (Uchida et al. 2000).
Renal failure has been described in the infantile variant of CoQ10 deficiency and was present in our patients (Rötig et al. 2000). The vulnerability of the kidney is more difficult to explain, but lipid peroxidation and altered mitochondrial function have been implicated in congenital nephrotic syndrome and glomerular proteinuria (Neale et al. 1994; Holthofer et al. 1999).
This is the first report of a molecular defect causing primary human CoQ10 deficiency and the initial description of defects in human PHB-polyprenyl transferase. Further studies are needed to understand the pathogenesis of this disease, and the detection of mutations in COQ2 in other patients will better define possible phenotypic variants in this condition. The availability of genetic testing will allow the initiation of early therapeutic intervention, even presymptomatically, in this otherwise life-threatening infantile encephalomyopathy responsive to CoQ10 supplementation.
Acknowledgments
We appreciate the generous cooperation of the patients and their relatives. The thoughtful comments of Dr. Eric Schon and excellent technical support of Ms. Saba Tadesse are acknowledged. The authors are supported by National Institutes of Health grant P01NS11766 and by grants from the Muscular Dystrophy Association and the Marriott Mitochondrial Disorder Clinical Research Fund.
Web Resources
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CoQ10 deficiency)
References
- Aure K, Benoist JF, Ogier de Baulny H, Romero NB, Rigal O, Lombes A (2004) Progression despite replacement of a myopathic form of coenzyme Q10 defect. Neurology 63:727–729 [DOI] [PubMed] [Google Scholar]
- Boitier E, Degoul F, Desguerre I, Charpentier C, François D, Ponsot G, Diry M, Rustin P, Marsac C (1998) A case of mitochondrial encephalomyopathy associated with a muscle coenzyme Q10 deficiency. J Neurol Sci 156:41–46 10.1016/S0022-510X(98)00006-9 [DOI] [PubMed] [Google Scholar]
- Brea-Calvo G, Rodriguez-Hernandez A, Fernandez-Ayala DJM, Navas P, Sanchez-Alcazar JA. Chemotherapy induces an increase in coenzyme Q10 levels in cancer cell lines. Free Radic Biol Med (in press) [DOI] [PubMed] [Google Scholar]
- Date H, Onodera O, Tanaka H, Iwabuchi K, Uekawa K, Igarashi S, Koike R, Hiroi T, Yuasa T, Awaya Y, Sakai T, Takahashi T, Nagatomo H, Sekijima Y, Kawachi I, Takiyama Y, Nishizawa M, Fukuhara N, Saito K, Sugano S, Tsuji S (2001) Early-onset ataxia with ocular motor apraxia and hypoalbuminemia is caused by mutations in a new HIT superfamily gene. Nat Genet 29:184–188 10.1038/ng1001-184 [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, Mirabella M, Spinazzola A, Crociani P, Silvestri G, Broccolini A, Tonali P, Di Mauro S, Servidei S (2001) Coenzyme Q10 reverses pathological phenotype and reduces apoptosis in familial CoQ10 deficiency. Neurology 57:515–518 [DOI] [PubMed] [Google Scholar]
- DiMauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348:2656–2668 10.1056/NEJMra022567 [DOI] [PubMed] [Google Scholar]
- Echtay KS, Winkler E, Klingenberg M (2000) Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature 408:609–613 10.1038/35046114 [DOI] [PubMed] [Google Scholar]
- Forsgren M, Attersand A, Lake S, Grunler J, Swiezewska E, Dallner G, Climent I (2004) Isolation and functional expression of human COQ2, a gene encoding a polyprenyl transferase involved in the synthesis of CoQ. Biochem J 382:519–526 10.1042/BJ20040261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gin P, Hsu AY, Rothman SC, Jonassen T, Lee PT, Tzagoloff A, Clarke CF (2003) The Saccharomyces cerevisiae COQ6 gene encodes a mitochondrial flavin-dependent monooxygenase required for coenzyme Q biosynthesis. J Biol Chem 278:25308–25316 10.1074/jbc.M303234200 [DOI] [PubMed] [Google Scholar]
- Gironi M, Lamperti C, Nemni R, Moggio M, Comi G, Guerini FR, Ferrante P, Canal N, Naini A, Bresolin N, DiMauro S (2004) Late-onset cerebellar ataxia with hypogonadism and muscle coenzyme Q10 deficiency. Neurology 62:818–820 [DOI] [PubMed] [Google Scholar]
- Holthofer H, Kretzler M, Haltia A, Solin ML, Taanman JW, Schagger H, Kriz W, Kerjaschki D, Schlondorff D (1999) Altered gene expression and functions of mitochondria in human nephrotic syndrome. FASEB J 13:523–532 [DOI] [PubMed] [Google Scholar]
- Kagan V, Serbinova E, Packer L (1990) Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophys Res Commun 169:851–857 10.1016/0006-291X(90)91971-T [DOI] [PubMed] [Google Scholar]
- Kalen A, Appelkvist EL, Chojnacki T, Dallner G (1990) Nonaprenyl-4-hydroxybenzoate transferase, an enzyme involved in ubiquinone biosynthesis, in the endoplasmic reticulum-Golgi system of rat liver. J Biol Chem 265:1158–1164 [PubMed] [Google Scholar]
- Lalani SR, Vladutiu GD, Plunkett K, Lotze TE, Adesina AM, Scaglia F (2005) Isolated mitochondrial myopathy associated with muscle coenzyme Q10 deficiency. Arch Neurol 62:317–320 10.1001/archneur.62.2.317 [DOI] [PubMed] [Google Scholar]
- Lamperti C, Naini A, Hirano M, De Vivo DC, Bertini E, Servidei S, Valeriani M, Lynch D, Banwell B, Berg M, Dubrovsky T, Chiriboga C, Angelini C, Pegoraro E, DiMauro S (2003) Cerebellar ataxia and coenzyme Q10 deficiency. Neurology 60:1206–1208 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Moreira MC, Barbot C, Tachi N, Kozuka N, Uchida E, Gibson T, Mendonca P, Costa M, Barros J, Yanagisawa T, Watanabe M, Ikeda Y, Aoki M, Nagata T, Coutinho P, Sequeiros J, Koenig M (2001) The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat Genet 29:189–193 10.1038/ng1001-189 [DOI] [PubMed] [Google Scholar]
- Musumeci O, Naini A, Slonim AE, Skavin N, Hadjigeorgiou GL, Krawiecki N, Weissman BM, Tsao CY, Mendell JR, Shanske S, De Vivo DC, Hirano M, DiMauro S (2001) Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology 56:849–855 [DOI] [PubMed] [Google Scholar]
- Naini A, Lewis VJ, Hirano M, DiMauro S (2003) Primary coenzyme Q10 deficiency and the brain. Biofactors 18:145–152 [DOI] [PubMed] [Google Scholar]
- Neale TJ, Ojha PP, Exner M, Poczewski H, Ruger B, Witztum JL, Davis P, Kerjaschki D (1994) Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest 94:1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki Y, Martí R, Copeland WC, Hirano M (2003) Site-specific mtDNA point mutations due to thymidine phosphorylase deficiency. J Clin Invest 111:1913–1921 10.1172/JCI200317828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasahara S, Engel AG, Frens D, Mack D (1989) Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc Natl Acad Sci USA 86:2379–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccio H, Koenig M (2002) Friedreich ataxia: a paradigm for mitochondrial diseases. Curr Opin Genet Dev 12:272–277 10.1016/S0959-437X(02)00298-8 [DOI] [PubMed] [Google Scholar]
- Quinzii CM, Kattah AG, Naini A, Akman HO, Mootha VK, DiMauro S, Hirano M (2005) Coenzyme Q deficiency and cerebellar ataxia associated with an aprataxin mutation. Neurology 64:539–541 [DOI] [PubMed] [Google Scholar]
- Rahman S, Hargreaves I, Clayton P, Heales S (2001) Neonatal presentation of coenzyme Q10 deficiency. J Pediatr 139:456–458 10.1067/mpd.2001.117575 [DOI] [PubMed] [Google Scholar]
- Rötig A, Appelkvist EL, Geromel V, Chretien D, Kadhom N, Edery P, Lebideau M, Dallner G, Munnich A, Ernster L, Rustin P (2000) Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet 356:391–395 10.1016/S0140-6736(00)02531-9 [DOI] [PubMed] [Google Scholar]
- Salviati L, Sacconi S, Murer L, Zaccello G, Franceschini L, Laverda AM, Basso G, Quinzii CM, Angelini C, Hirano M, Naini A, Navas P, DiMauro S, Montini G (2005) Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: a CoQ10-responsive condition. Neurology 65:606–608 [DOI] [PubMed] [Google Scholar]
- Sobreira C, Hirano M, Shanske S, Keller RK, Haller RG, Davidson E, Santorelli FM, Miranda AF, Bonilla E, Mojon DS, Barreira AA, King MP, DiMauro S (1997) Mitochondrial encephalomyopathy with coenzyme Q10 deficiency. Neurology 48:1238–1243 [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G (2004) Metabolism and function of coenzyme Q. Biochim Biophys Acta 1660:171–199 [DOI] [PubMed] [Google Scholar]
- Uchida N, Suzuki K, Saiki R, Kainou T, Tanaka K, Matsuda H, Kawamukai M (2000) Phenotypes of fission yeast defective in ubiquinone production due to disruption of the gene for p-hydroxybenzoate polyprenyl diphosphate transferase. J Bacteriol 182:6933–6939 10.1128/JB.182.24.6933-6939.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maldergem L, Trijbels F, DiMauro S, Sindelar PJ, Musumeci O, Janssen A, Delberghe X, Martin JJ, Gillerot Y (2002) Coenzyme Q-responsive Leigh’s encephalopathy in two sisters. Ann Neurol 52:750–754 10.1002/ana.10371 [DOI] [PubMed] [Google Scholar]
- Yokota T, Igarashi K, Uchihara T, Jishage K, Tomita H, Inaba A, Li Y, Arita M, Suzuki H, Mizusawa H, Arai H (2001) Delayed-onset ataxia in mice lacking alpha-tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc Natl Acad Sci USA 98:15185–15190 10.1073/pnas.261456098 [DOI] [PMC free article] [PubMed] [Google Scholar]