Abstract
OBJECTIVE
To test the effectiveness of a brief intervention in the reduction of prenatal alcohol consumption by women when a partner is included.
METHODS
Randomized trial of a single session brief intervention given by the study nurse or principal investigator for 304 pregnant women and their partners. The women had positive T-ACE (Tolerance, Annoyed, Cut down, Eye-opener, analcohol screening test) results and were at risk for alcohol consumption while pregnant. All completed initial diagnostic and postpartum interviews.
RESULTS
Fewer than 20% of participants (median 11.5 weeks of gestation) were abstinent at study enrollment, averaging more than 1.5 drinks per episode. Nearly 30% had 2 or more drink sat a time while pregnant. Prenatal alcohol use declined in both the treatment and control groups after study enrollment, based on a 95% follow-up rate. Factors associated with increased prenatal alcohol use after randomization included more years of education, extent of previous alcohol consumption, and temptation to drink in social situations. Brief interventions for prenatal alcohol reduced subsequent consumption most significantly for the women with the highest consumption initially (regression coefficient, b = –0.163, standard error (b) = 0.063, P < .01). Moreover, the effects of the brief intervention were significantly enhanced when a partner participated (b = –0.932, standard error (b) = 0.468), P < .05).
CONCLUSION
Pregnant women with the highest levels of alcohol use reduced their drinking most after a brief intervention that included their partners. Recommendations include consistent screening for prenatal alcohol use followed by diagnostic assessment when indicated, and if confirmed by other studies, a patient-partner brief intervention for the heaviest drinkers.
LEVEL OF EVIDENCE
I
Maternal prenatal alcohol use is one of the leading preventable causes of birth defects, mental retardation, and neurodevelopmental disorders in the United States.1 Despite accumulating evidence that prenatal alcohol consumption at levels less than one drink per day can adversely affect fetal growth and development, pregnant women continue to drink.2,3 The prevalence of any alcohol use among pregnant women was 12.8% in 1999, with 6% of women reporting frequent (defined as more than 7 drinks per week) and binge (defined as 5 or more drinks per episode) drinking.4 Although this is an improvement over the 1988 baseline rate of 21% of pregnant women consuming alcohol, neither the Healthy People 2000 nor the Healthy People 2010 goal of 94% abstinence from alcohol during pregnancy would be satisfied.5,6
Abstinence during pregnancy is the recommendation of both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists to pregnant and preconceptional women.7 Thus, early identification and modification of prenatal alcohol use are highly desirable, particularly because past drinking predicts drinking levels during pregnancy.8 The T-ACE (Tolerance, Annoyed, Cut down, Eye-opener) is a validated screening instrument for prenatal alcohol use that may facilitate early recognition.9,10 Although initial treatment research focused on the pregnant drinker with severe alcohol problems, modification of low-to-moderate (one drink a day or less) prenatal consumption may provide the most benefit since these levels of consumption are more commonly reported.11,12 For example, a study of 361 highly disadvantaged gravidas who acknowledged having 1.3 drinks per week antenatally were actually drinking at rather higher levels.13 In fact, there is no universally safe level of prenatal alcohol use, which has led to the recommendations of abstinence.14
In general, expectant fathers or partners are not routinely screened for health problems or behaviors that could impact the pregnant woman’s health habits. Yet, several studies support the expectant father or partner as an influential modifier of prenatal behaviors. A convenience sample of 153 pregnant women in their third trimester found that social support was significantly related to reduction of alcohol use in pregnancy.15 Two European studies of prenatal cigarette smoking identified the partner’s smoking habits as being one of the most powerful predictors of smoking cessation by the pregnant woman.16,17
The purpose of this randomized trial was to test the effectiveness of a brief intervention enhanced by including a partner chosen by a pregnant woman. The partner could be her spouse, father of the child, or any other supportive adult who would be knowledgeable about her health habits. We hypothesized that, although both groups of pregnant women would demonstrate reductions in prenatal alcohol use, those randomized to the brief intervention with a partner would have greater declines in antenatal alcohol consumption.
SUBJECTS AND METHODS
Potential participants completed the Health and Habits Survey, which contained questions about diet, smoking, exercise, stress, and usual drinking, and the T-ACE, a 4-item alcohol-screening instrument. The Health and Habits Survey was given to patients initiating prenatal care at 1 of 3 obstetric practices (clinic, faculty, or private group affiliate) of the Brigham and Women’s Hospital in Boston, Massachusetts. E-mail and other study announcements also invited study inquiries, and the screening survey was made available at the time of inquiry.
Research assistants evaluated potential participants for 4 inclusion criteria. The first criterion was a positive T-ACE, with a total score of 2 or more. The T-ACE asks 4 questions that give the assessment its name: T, how many drinks does it take to make you feel high? (Tolerance); A, have people ever annoyed you by criticizing your drinking? (Annoyed); C, have you ever felt you ought to cut down on your drinking (Cut down); and E, have you ever had a drink first thing in the morning to steady your nerves or get rid of a hangover (Eye-opener). The tolerance question is given 2 points if the respondent reports needing more than 2 drinks, and affirmative replies to the A, C, and E questions are each given one point. The second criterion was being at risk for prenatal alcohol use, which was defined as any alcohol consumption in the 3 months before study enrollment (while pregnant), or consumption of at least one drink per day in the 6 months before study enrollment, or drinking during a previous pregnancy. The third criterion was gestation less than 28 weeks and intention to carry pregnancy to term. The fourth criterion was agreement to study terms, which included randomization to treatment by computer assignment, postpartum follow-up interview, selection of a partner who would participate in a diagnostic interview, the brief intervention if so randomized, and postpartum interview. Subjects were informed of their treatment assignment at the time of study enrollment, before the diagnostic interview.
Study exclusion criteria were 1) current treatment for alcohol or drug abuse, or substance abuse–related medical illness, 2) current physical dependence on alcohol requiring medically supervised detoxification, 3) current use of opiates, cocaine, or other illicit substances, 4) inability to complete study questionnaires, and 5) intention to terminate pregnancy before term gestation.
Sample size calculations for the trial were based on 95% confidence level (α = 0.05), 90% power, 1:1 ratio of treatment to control subjects, and the expectation that 50% of the control group would become abstinent, based on the spontaneous rate of abstinence reported for pregnant women.18 The rate of abstinence in the brief intervention group was estimated to be 70%, based on the rate reported from a previous study of brief intervention for prenatal alcohol use.19 Thus, the total number of subjects needed would be 268, without attrition, and 295, with 10% attrition. Our goal was to enroll 300 subjects.
At the diagnostic interview given by research assistants, pregnant participants completed the 1) Alcohol Timeline Followback, to obtain estimates of their daily drinking for the 6 months before study enrollment;20 2) Alcohol Abstinence Self-Efficacy scale, to measure their evaluations of their perceived temptation to drink and their efficacy to abstain in 20 common situations;21 and 3) Healthy Pregnancy Facts, a series of 7 statements about healthy habits during pregnancy that the respondent was asked to judge as true or false, among other instruments.
Separately, the partners met with research assistants to complete 1) the Health and Habits Survey, already described; 2) National Institute on Alcohol Abuse and Alcoholism quantity-frequency questions, 9 questions about personal use of beer, wine, whiskey, gin, or other spirits in the previous 30 days;22 3) collateral report, the National Institute on Alcohol Abuse and Alcoholism quantity-frequency questions about the partner’s alcohol use in the past 90 days; and 4) Healthy Pregnancy Facts, a series of 7 statements about healthy habits during pregnancy that the respondent was asked to judge as true or false.
The brief intervention was then given to the randomly assigned couple by 1 of 2 nurse practitioners or the principal investigator according to the following structure: 1) knowledge assessment with feedback, 2) contracting and goal setting, 3) behavioral modification, and 4) summary. The single-session brief intervention was selected because of its efficiency and prior history of success, but was enhanced in this study by including the partner.19 The interventionists, all experienced clinicians with at least a master’s degree, were trained and directly observed in the delivery of the brief intervention. Their brief intervention summaries and other notes were continuously reviewed for treatment consistency. Each intervention took an average of 25 minutes to complete. Brief interventions were not audio taped because of concerns about participant acceptability.
Knowledge assessment with feedback1 began with a review of the Healthy Pregnancy Facts knowledge measure completed by both the subject and her partner. Questions were answered and any misapprehensions were discussed. The subject’s actual alcohol consumption was not discussed in the presence of her partner, unless she volunteered the information. In the next step of goal setting and contracting,2 the subject was asked to describe her prenatal drinking goal (eg, abstinence), and the rationale for her choice was explored. The couple was informed that maternal abstinence from alcohol was the most prudent choice during pregnancy. They were asked if either the subject or the couple had made any lifestyle changes because of her pregnancy (eg, work schedule). The behavioral modification3 portion consisted of asking the subject to identify situations or circumstances when she might be tempted to drink alcohol (eg, at a wedding) and to then list some alternative behaviors (eg, having some food instead). The partner was asked to describe ways in which he or she had modified or made plans to change behaviors that could offer support to the pregnant woman, such as drinking less, socializing differently, or doing more at home. The content of the brief intervention was summarized4 on a no-carbon-required form, and the couple was given the original summary. The brief intervention was timed and the interventionist was asked to record any relevant impressions on a separate sheet after the intervention, in addition to the summary form.
At the postpartum follow-up interview, subjects completed the 1) Alcohol Timeline Followback for alcohol consumption from the time of study enrollment until delivery, and 2) Alcohol Abstinence Self-Efficacy scale, already described. At the postpartum interview, partners provided 1) a collateral report on the subject’s use of alcohol since study enrollment using the National Institute on Alcohol Abuse and Alcoholism quantity-frequency questions, 2) follow-up Health and Habits Survey, to assess changes in health habits by the partner since enrollment, and 3) National Institute on Alcohol Abuse and Alcoholism quantity-frequency questions about personal consumption of beer, wine, whiskey, gin, or other spirits since study enrollment. Whenever possible, the subjects and partners completed the follow-up interviews with research assistants who did not administer their diagnostic interviews.
Subjects who completed the diagnostic interview (control) or diagnostic interview with brief intervention (treatment) received an honorarium of $50.00 and $100.00 for the postpartum interview. Support partners received an honorarium of $25.00 for each of the diagnostic and postpartum interviews.
This study was reviewed and approved by the Institutional Review Board of the Brigham and Women’s Hospital. In addition, a Certificate of Confidentiality for the project was granted by the Department of Health and Human Services.
Data were analyzed using univariate and multivariable techniques to compare the treatment (brief intervention) and control (diagnostic interview only) groups before and after study enrollment with SAS 8.2 (SAS Institute, Cary, NC). Descriptive results are reported as percentages and means. Baseline patient demographic and behavioral characteristics were compared between the 2 study arms using Wilcoxon or Fisher exact tests.
Ordinary least-squares regression models were used to evaluate the effect of the brief intervention on 3 dependent variables: drinks per drinking day (quantity), percentage of drinking days (frequency), and a combined quantity-frequency measure subsequent to study enrollment. To control confounding and reduce variability, all regression models included demographic variables, history of prior drinking, temptation and confidence in managing temptation to drink in a variety of circumstances, use of cigarettes, and high-risk pregnancy status, in addition to the primary predictor indicating treatment or control status. The first model was an intent-to-treat analysis of all 304 subjects. The second model added an interaction term between treatment status and alcohol use at enrollment to the first model to investigate whether women with the more severe drinking problems might benefit the most from the intervention.23 The third model was a brief intervention efficacy analysis that specifically examined the impact of the support partner’s participation in the treatment. Multiple imputation, with 5 imputations, was used to manage missing data.24 All analyses were replicated with mean substitution to verify the findings from the multiple imputation.
RESULTS
Figure 1 summarizes study subject flow. Between February 2000 and September 2002, 2,927 Health and Habits screening surveys were returned. Eighty-nine percent of those screened were initiating prenatal care at the Brigham and Women’s Hospital. The rest responded to an e-mail or other study announcement. The majority (78%) of the 802 women with T-ACE-positive screening surveys (27.4% of 2,927) indicated their initial willingness to be contacted and screened for study eligibility. Of the 399 successfully contacted, 86.5% met study eligibility criteria, and 88% of those eligible agreed to participate in the study, resulting in a total enrollment of 304 pregnant women and their partners who were randomized to either the enhanced brief intervention with diagnostic interview or diagnostic interview only. Postpartum follow-up data were available for 95% of the subjects enrolled. Despite all efforts to accommodate partners, 3% were ultimately unable to participate in any part of the study.
Fig. 1. Progress through stages of study.
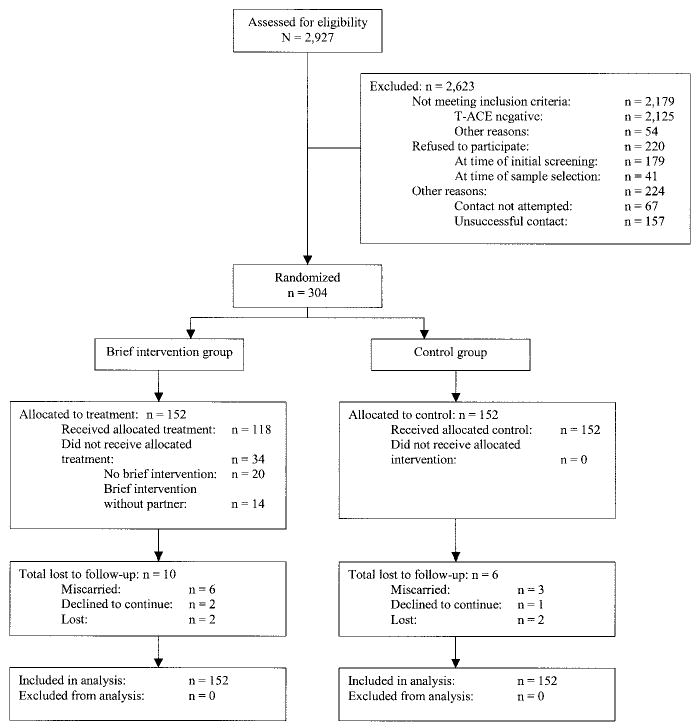
Chang. Randomized Trial for Prenatal Alcohol Use. Obstet Gynecol 2005.
The demographic and clinical characteristics of the study sample are summarized in Table 1. Subjects were predominantly white (78.6%) and married (80.5%), with a median age of 31.4 years and a median education level of a 4-year college degree or equivalent. Median income for home ZIP code was $55,357; the average median household income for Massachusetts in the study time period was $50,587.25 Most subjects selected their husbands or the biological fathers of the child (87%) to be involved in the study. Both treatment and control groups had similar results on the Alcohol Abstinence Self-Efficacy measure of confidence to abstain from drinking alcohol and temptation to drink in 20 common situations “at the present time.” Both groups reported the greatest temptation to drink in social situations in comparison to other circumstances.
Table 1.
Demographic and Clinical Profile at Study Enrollment
| Treatment Group (n = 152) | Control Group (n = 152) | |
|---|---|---|
| Demographic variables | ||
| Race | ||
| African American (%) | 8.6 | 6.6 |
| White (%) | 78.4 | 78.8 |
| Other (%) | 13.0 | 14.6 |
| Median age (y) | 32.0 | 30.7 |
| Married or committed relationship (%) | 82.2 | 78.8 |
| Median years of education | 16 | 16.3 |
| Median income for home zip code ($) | 54,676 | 55,700 |
| Obstetric history | ||
| Expecting first child (%) | 43.4 | 40.4 |
| Median gestation at enrollment (wk) | 11.0 | 12.0 |
| History of ob-gyn problems (%) | 26.8 | 21.5 |
| Infant birth weight (g) | 3,345 | 3,345 |
| Alcohol use variables | ||
| T-ACE positive (%) | 100 | 100 |
| Tolerance, median no. of drinks | 3 | 3 |
| Annoy (%) | 5.3 | 7.9 |
| Cut down (%) | 23.2 | 16.5 |
| Eye-opener (%) | 1.3 | 2.6 |
| Alcohol Abstinence Self-Efficacy Scale | ||
| Median confidence, craving | 25 | 25 |
| Median confidence, physical | 25 | 25 |
| Median confidence, negative affect | 25 | 25 |
| Median confidence, social | 24 | 24 |
| Median temptation, craving | 5 | 5 |
| Median temptation, physical | 5 | 5 |
| Median temptation, negative affect | 5 | 5 |
| Median temptation, social | 9 | 9 |
| Prepregnancy alcohol use | ||
| Mean % days drinking | 20.9 | 20.3 |
| Mean drinks per drinking day (n) | 1.85 | 1.82 |
| Median % years lifetime regular use | 3.95 | 0 |
| Prenatal alcohol use at enrollment | ||
| Mean % days drinking | 5.4 | 5.0 |
| Mean drinks per drinking day (n) | 1.6 | 1.6 |
| Partner information | ||
| Median age (y) | 32.4 | 32.5 |
| Relationship to subject | ||
| Male husband or biological father (%) | 89.6 | 85.7 |
| Female partner (%) | 8.3 | 11.6 |
| Other (%) | 2.1 | 2.7 |
| Smokes cigarettes (%) | 11.1 | 9.5 |
| Exercises regularly (%) | 59.0 | 58.5 |
| Experiences stress (%) | 65.3 | 59.9 |
T-ACE, Tolerance, Annoyed, Cut down, Eye-opener, an alcohol screening test.
Subjects were asked to estimate the number of years they had been consuming alcohol on a regular basis. Overall, participants estimated a mean of 3.7 years of regular alcohol use, with no statistically significant differences between the 2 groups. The number of years was then converted to a percentage of the subject’s lifetime to allow for ease of comparison between subjects.
The alcohol consumption of the subjects can be summarized for 3 time periods: prepregnancy (average of 79 days), prenatal before study enrollment (average of 104 days), and then prenatal subsequent to study enrollment (average of 158 days). There were no statistically significant differences in the amount or frequency of prepregnancy alcohol consumption when the control and treatment groups were compared. On average, the groups consumed alcohol on 20% of the prepregnancy days and had a mean of 1.8 drinks per episode. Less than 10% were abstinent from alcohol in the time period covered. Once pregnant, many of the women spontaneously decreased the frequency of their alcohol consumption to a mean of 5% drinking days, but fewer than 20% were abstinent. The groups averaged more than one and a half drinks per episode, but nearly 30% of subjects in each group had 2 or more drinks at a time while pregnant. Alcohol consumption after study enrollment declined further in both groups to a mean (μ) average of 2% of days (treatment, μ = 1.9%, versus control, μ = 2.0%) and less than half a drink per episode until the time of delivery (treatment, μ = 0.39, versus control, μ = 0.40).
Since both the treatment and control groups demonstrated overall reduced alcohol consumption once enrolled, the impact of the brief intervention on different levels of prenatal consumption at enrollment was evaluated. The interaction between the brief intervention and prenatal alcohol consumption was significant (regression coefficient, b = –0.163, standard error [SE] (b) = 0.063, P < .01), indicating that the brief intervention was more effective in reducing frequency of consumption among women who drank more at the time of study enrollment. For example, a subject who reported drinking on 15% of days when she enrolled in the study would be expected to reduce drinking to 5% of days if she received only the diagnostic interview. If she received the brief intervention, her drinking would be reduced to 3% of days (Fig. 2).
Fig. 2. Predicted percentage of drinking days in the intention-to-treat model.
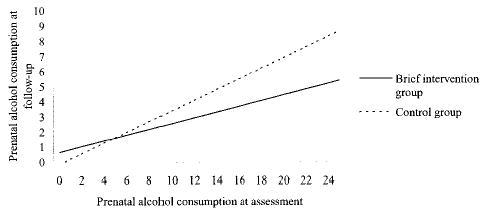
Chang. Randomized Trial for Prenatal Alcohol Use. Obstet Gynecol 2005.
Several other variables were found to increase the risk of prenatal alcohol consumption after enrollment in the intention-to-treat model. Three variables increased the frequency of consumption: 1) amount of prenatal alcohol use before study enrollment (b = 0.354, SE (b) = 0.047, P < .001), 2) extent of education (b = 0.021, SE (b) = 0.008, P < .01), and 3) number of years of regular alcohol use (b = 0.355, SE (b) = 0.172, P < .05). On the other hand, increased confidence in managing temptation to drink in social situations reduced the frequency of consumption (b = –0.025, SE (b) = 0.008, P < .001). Temptation to drink in social situations increased the risk of more drinks per episode (b = 0.002, SE (b) = 0.001, 1.004, P < .05). Table 2 summarizes the results of the intention-to-treat analysis with the interaction term.
Table 2.
Prenatal Alcohol Consumption After Randomization to Treatment (n = 304)*
| Drinks/Drinking Day
|
Percentage Drinking Days
|
Combined
|
||||
|---|---|---|---|---|---|---|
| b | SE (b) | b | SE (b) | b | SE (b) | |
| Treatment status (BI) | 0.014 | 0.087 | 0.802 | 0.587 | 0.645 | 0.500 |
| Prenatal alcohol consumption at enrollment | −0.004 | 0.031 | 0.354 | 0.047† | 0.052 | 0.018‡ |
| Interaction between BI and extent of prenatal alcohol use at enrollment | −0.016 | 0.042 | −0.163 | 0.063‡ | −0.035 | 0.022 |
| Race | ||||||
| African American | −0.092 | 0.119 | −1.833 | 1.033 | −1.222 | 0.920 |
| Other | −0.136 | 0.090 | −0.761 | 0.806 | −0.829 | 0.733 |
| Expecting first child | −0.018 | 0.065 | −0.719 | 0.581 | −0.683 | 0.519 |
| Temptation to drink in social situations (10×) | 0.002 | 0.001§ | −0.008 | 0.008 | −0.001 | 0.007 |
| Confidence to manage social situations (10×) | 0.000 | 0.001 | −0.025 | 0.008† | 0.023 | 0.007† |
| Education in months (10×) | −0.001 | 0.001 | 0.021 | 0.008‡ | 0.017 | 0.008§ |
| Percentage of years drinking alcohol to intoxication | 0.056 | 0.020‡ | 0.355 | 0.172§ | 0.589 | 0.154‡ |
| R2 | 0.146 | 0.350 | 0.231 | |||
regression coefficient; SE (b), standard error of b; BI, brief intervention.
Other factors controlled for include marital status, age in years, cigarette smoker, and high-risk pregnancy.
P < .001.
P < .01.
P < .05.
An efficacy (subgroup) analysis evaluating the effect of partner involvement in the brief intervention compared the drinking outcomes of the 118 subjects whose partners participated in the brief intervention to those (n = 14) whose partners did not. Twenty brief interventions did not take place. The brief intervention was more effective for the heavier-drinking subject when her partner was involved, when drinking was measure by percentage of days drinking (b = –0.867, SE (b) = 0.419, P < .05) and the combined measure of drinking (b = –0.932, SE (b) = 0.468, P < .05). Other subject factors associated with subsequent prenatal alcohol use were similar to those in the intent-to-treat model and are listed in Table 3.
Table 3.
When the Support Partner Participates: Subsequent Prenatal Alcohol Use (n = 132)*
| Drinks/Drinking Day
|
Percentage Drinking Days
|
Combined
|
||||
|---|---|---|---|---|---|---|
| b | SE (b) | b | SE (b) | b | SE (b) | |
| Support partner participated in the BI | −0.037 | −0.212 | 1.699 | 1.297 | 2.023 | 1.247 |
| Subject prenatal alcohol use at enrollment | −0.120 | 0.194 | 1.092 | 0.413† | 0.955 | 0.468‡ |
| Interaction between support partner participation in the BI and subject’s prenatal alcohol use at enrollment | 0.066 | 0.206 | −0.867 | 0.419‡ | −0.932 | 0.469‡ |
| Race | ||||||
| African American | −0.127 | 0.197 | −0.591 | 1.367 | −1.074 | 1.400 |
| Other | −0.328 | 0.130† | 0.073 | 0.935 | −0.233 | 0.918 |
| Expecting first child | −0.046 | 0.091 | −0.713 | 0.637 | −0.739 | 0.670 |
| Temptation to drink in social situations (10×) | 0.004 | 0.001‡ | −0.013 | 0.010 | −0.009 | 0.010 |
| Confidence to manage social situations (10×) | 0.002 | 0.001 | −0.038 | 0.009§ | −0.033 | 0.009§ |
| Subject education in months (10×) | −0.001 | 0.002 | 0.008 | 0.011 | 0.008 | 0.011 |
| Percentage years subject drinking alcohol to intoxication | 0.090 | 0.030† | 0.642 | 0.220† | 0.733 | 0.213§ |
| R2 | 0.288 | 0.422 | 0.327 | |||
regression coefficient; SE (b), standard error of b; BI, brief intervention.
Other factors controlled for include marital status, age in years, cigarette smoker, and high-risk pregnancy.
P < .01.
P < .05.
P < .001.
DISCUSSION
In this study of 304 pregnant women with 95% postpartum follow-up, fewer than 20% of subjects were abstinent at 11.5 weeks of gestation, and nearly 30% had 2 or more drinks at a time when enrolled. The main findings are that brief interventions for prenatal alcohol use are more effective in reducing subsequent consumption for women who are drinking more often when it is administered (P < .01). Moreover, the effects of the brief intervention are significantly enhanced when a support partner of the woman’s choice also participates in the brief intervention (P < .05).
Prenatal alcohol use declined in both the treatment and control groups after study enrollment. Reductions in alcohol use among pregnant women in the control group is a finding consistent with other studies of women and may be explained by subject reactivity to research protocols, regression to the mean, or reporting bias.13,26,27,28 A diagnostic interview is the first step in most treatment efforts.
Factors associated with increased prenatal alcohol consumption in this sample were identified. More education, temptation to drink in social situations, previous drinking history of more consumption for longer periods of time, and more prenatal drinking at enrollment all predicted more antenatal alcohol use, consistent with previous studies.14,29 On the other hand, more confidence in managing temptation to drink in social situations was associated with less drinking. However, it is also noted that all subjects expressed maximum confidence in managing other risk situations for drinking alcohol and yet, fewer than 20% were abstinent at the time of study enrollment. The high rates of confidence may reflect some limitations of the Alcohol Abstinence Self-Efficacy scale or the possibility that participants simply overestimated their efficacy in managing such risk situations.
Limitations to the generalizability of study findings include the possibility of assembly bias, despite the nearly 90% rate of enrollment. For example, particularly motivated women may have been inclined to participate. Since they were able to include a partner, their circumstances may have been more stable or supportive, factors that would improve any therapeutic outcome. Study subjects were well educated and had median incomes somewhat higher than average, 2 characteristics associated with increased risk of prenatal drinking. They may have underreported alcohol consumption after study enrollment, when the salience of drinking may have been increased. Although it has long been assumed that women systematically underreport prenatal alcohol use, recent studies have demonstrated that, not only do pregnant women provide valid information about their drinking, but their self-reports of drinking exceed those given by collateral reporters.30,31 Interviewers were not blinded to treatment assignment, which would have rendered the study logistically impossible, but data were gathered using structured measures at all times and, when possible, different research assistants gave the diagnostic and follow-up interviews. Treatment fidelity was assessed by reviewing the brief intervention summary notes, consistent with the medical model of documentation, as opposed to review of audio tapes.
Several recommendations for future directions might be considered. First, consistent screening for prenatal alcohol use with a validated instrument embedded in a patient information form may provide valuable information to the clinician. Of the 2,927 pregnant women screened, 27.4% had positive alcohol screens, with a median tolerance to 3 drinks containing alcohol. Second, a diagnostic interview about alcohol use appears to result in reduced consumption subsequently. Thus, screening and assessment may be the most parsimonious approach to the identification and management of pregnancy risk drinking. Third, a brief intervention involving not only the pregnant woman but also a partner of her choice may be especially effective for those women who are drinking more prenatally. Fourth, social situations appear to the most “risky” for prenatal alcohol consumption, so that techniques to increase management of this specific risk are needed.
Abstinence from alcohol is the most prudent course for pregnant women, because there is no universally safe limit. The absence of a safe limit may lead some patients and their physicians to interpret that lower levels of drinking are otherwise acceptable and thus may account for the low rates of abstinence in this sample at enrollment.32 However, the overall decline in consumption in the participants after study enrollment suggests that screening, assessment, and intervention with a partner can effectively reduce their antenatal alcohol use and minimize fetal risk.
Footnotes
This study was supported by grants R01 AA12548 (G.C.) and K24 AA 00289 (G.C.) from the National Institute on Alcohol Abuse and Alcoholism.
References
- 1.American Academy of Pediatrics. Committee on Substance Abuse and Committee on Children With Disabilities. Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106:358–61. [PubMed] [Google Scholar]
- 2.Wong EV, Kenwrick S, Willems P, Lemmon V. Mutations in the cell adhesion molecule L1 cause mental retardation. Trends Neurosci. 1995;18:168–172. doi: 10.1016/0166-2236(95)93896-6. [DOI] [PubMed] [Google Scholar]
- 3.Charness ME, Safran RM, Perides G. Ethanol inhibits neural cell-cell adhesion. J Biol Chem. 1994;269:9304–9. [PubMed] [Google Scholar]
- 4.Alcohol use among women of childbearing age, United States, 1991–1999 [published erratum appears in MMWR Morb Mortal Wkly Rep 2002;51:308]. MMWR Morb Mortal Wkly Rep 2002;51:273–6. [PubMed]
- 5.U.S. Department of Health and Human Services. Appendix A. In: Healthy people 2000 midcourse review and 1995 revisions. Sudbury (MA): Jones & Bartlett Publishers; 1995. p. 228.
- 6.U.S. Department of Health and Human Services. Objectives for improving health. Part B. In: Healthy people 2010. 2nd ed. Washington (DC): U.S. Government Printing Office; 2000. p. 16–43.
- 7.Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–9. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- 8.Russell M, Martier SS, Sokol RJ, Mudar P, Bottoms S, Jacobson S, et al. Screening for pregnancy risk-drinking. Alcohol Clin Exp Res. 1994;18:1156–61. doi: 10.1111/j.1530-0277.1994.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang G. Alcohol-screening instruments for pregnant women. Alcohol Res Health. 2001;25:204–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Chang G, Wilkins-Haug L, Berman S, Goetz MA, Behr H, Hiley A. Alcohol use: improving identification. Obstet Gynecol. 1998;91:892–8. doi: 10.1016/s0029-7844(98)00088-x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW, Kaplan MG. Maternal recall of alcohol, cocaine, and marijuana use during pregnancy. Neurotoxicol Teratol. 1991;13:535–40. doi: 10.1016/0892-0362(91)90062-2. [DOI] [PubMed] [Google Scholar]
- 12.Hankin J, McCaul ME, Heussner J. Pregnant, alcohol-abusing women. Alcohol Clin Exp Res. 2000;24:1276–86. [PubMed] [Google Scholar]
- 13.Jacobson JL, Jacobson SE. Prenatal alcohol exposure and neurobehavioral development: where is the threshold? Alcohol Health Res World. 1994;18:30–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Stratton K, Howe C, Battaglia F, editors. Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. Washington (DC): National Academy Press; 1996.
- 15.Coleman MA, Coleman NC, Murray JP. Mutual support groups to reduce alcohol consumption. Health Mark Q. 1990;7:47–63. doi: 10.1300/J026v07n03_05. [DOI] [PubMed] [Google Scholar]
- 16.Olsen J. Predictors of smoking cessation in pregnancy. Scand J Soc Med. 1993;21:197–202. doi: 10.1177/140349489302100309. [DOI] [PubMed] [Google Scholar]
- 17.Waterson EJ, Evans C, Murray-Lyon IM. Is pregnancy a time for changing drinking and smoking patterns for fathers as well as mothers? Br J Addict. 1990;85:389–96. doi: 10.1111/j.1360-0443.1990.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith IE, Lancaster JS, Moss-Wells S. Identifying high-risk pregnant drinkers: biological and behavioral correlates of continuous heavy drinking during pregnancy. J Stud Alcohol. 1987;48:304–9. doi: 10.15288/jsa.1987.48.304. [DOI] [PubMed] [Google Scholar]
- 19.Chang G, Wilkins-Haug L, Berman S, Goetz MA. Brief intervention for alcohol use in pregnancy: a randomized trial. Addiction. 1999;94:1499–1508. doi: 10.1046/j.1360-0443.1999.941014996.x. [DOI] [PubMed] [Google Scholar]
- 20.Sobell LC, Sobell MB. Timeline followback: a technique for assessing self-reported alcohol consumption. In: Allen JP, Litten RZ, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa (NJ): Humana Press; 1992. p. 41–72.
- 21.DiClemente CC, Carbonari JP, Montgomery RPG, Hughes SO. The alcohol abstinence self-efficacy scale. J Stud Alcohol. 1994;55:141–8. doi: 10.15288/jsa.1994.55.141. [DOI] [PubMed] [Google Scholar]
- 22.NIAAA Quantity-Frequency Questions. In: Allen JP, Columbus M, editors. Assessing alcohol problems, a guide for clinicians and researchers. Bethesda (MD): National Institutes of Health Publication No. 95-3745; 1995. p. 460–1.
- 23.Jaccard J, Turrisi R. Interaction effects in multiple regression. 2nd ed. Thousand Oaks (CA): Sage Publications; 2003.
- 24.Rubin DB. Multiple imputation for nonresponse in surveys. New York (NY): J. Wiley and Sons; 1987.
- 25.DeNavas WC, Cleveland R, Webster BH Jr. Income in the United States: 2002. U.S. Census Bureau, Current Population Reports P60-221. Washington (DC): U.S. Government Printing Office; 2003. Available at: http://www.census.gov/prod/2003pubs/p60-221.pdf Retrieved January 26, 2005.
- 26.Clifford PR, Maisto SA. Subject reactivity effects and alcohol treatment outcome research. J Stud Alcohol. 2000;61:787–93. doi: 10.15288/jsa.2000.61.787. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization Brief Intervention Study Group. A cross-national trial of brief interventions with heavy drinkers. Am J Public Health. 1996;86:948–955. doi: 10.2105/ajph.86.7.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers. JAMA. 1997;277:1039–45. [PubMed] [Google Scholar]
- 29.Ebrahim SH, Luman ET, Floyd RL, Murphy CC, Bennett EM, Boyle CA. Alcohol consumption by pregnant women in the United States during 1988–1995. Obstet Gynecol. 1998;92:187–92. doi: 10.1016/s0029-7844(98)00205-1. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–25. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- 31.Chang G, Goetz MA, Wilkins-Haug L, Berman S. Prenatal alcohol consumption: self versus collateral report. J Subst Abuse Treat. 1999;17:85–9. doi: 10.1016/s0740-5472(98)00053-1. [DOI] [PubMed] [Google Scholar]
- 32.Loop KQ, Nettleman MD. Obstetrical textbooks: recommendations about drinking during pregnancy. Am J Prev Med. 2002;23:136–8. doi: 10.1016/s0749-3797(02)00466-x. [DOI] [PubMed] [Google Scholar]


