ABSTRACT
We describe a 71-year-old woman who complained of a 1-year history of double vision when looking to the left, numbness over the right cheek, intermittent tinnitus, and gradually increasing unsteadiness when walking. Computed tomography and magnetic resonance imaging revealed a cholesterol granuloma at the right pyramidal apex anterior to the internal auditory canal and a slight compression of the brainstem on the ipsilateral side. For surgical removal we used the transtemporal approach instead of the trans-sphenoidal approach to obtain better control over the internal carotid artery. To avoid the problems of stenting, the resulting dead space was obliterated with fat. We discuss the essential preoperative imaging, controversies in choosing the appropriate surgical approach, and developments in treatment.
Keywords: Cholesterol granuloma, skull base, surgical approach
The petrous apex is a truncated pyramid forming the medial portion of the temporal bone. The base is bounded by the bony labyrinth and the internal carotid artery (ICA) anteriorly. Its superior surface is formed by the middle cranial fossa, Meckel's cave, and the ascending ICA. Its posterior surface is formed by the posterior cranial fossa and Dorello's canal transmitting cranial nerve VI. Its inferior surface is formed by the jugular bulb and the inferior petrosal sinus. The petrous apex is marrow-filled in 84%, pneumatized in 9%, and sclerotic in 7% of temporal bones.1
The petrous apex cannot be examined directly; therefore, imaging plays a primary role in the evaluation of lesions in this area. Most petrous apex lesions are readily characterized as either surgical (neoplastic lesions, inflammatory complications of air cell disease) or as incidental nonoperative findings (asymmetric fatty marrow, trapped fluid).2
A cholesterol granuloma is a foreign body giant cell reaction to cholesterol deposits, with associated fibrosis and vascular proliferation. The primary pathogenic mechanism in the development of cholesterol granulomas within the temporal bone seems to be occlusion of the air cell system.3 Obstruction of these cells leads to decreased intracavity pressure, mucosal inflammation and edema, angiogenesis, and subsequent rupture of blood vessels. Stagnate hemorrhagic contents undergo catabolism to hemoglobin breakdown products. The subsequent accumulation of cholesterol crystals serves as a strong irritant to surrounding tissue and serves to repeat and intensify the cycle.4
Cholesterol granulomas may occur anywhere within the pneumatized spaces of the temporal bone.3 Cholesterol granulomas of the petrous apex are uncommon because the petrous apex is pneumatized in only 30% of temporal bones.5 The prevalence of acoustic neuromas is estimated to be 30 times that of cholesterol granulomas of the petrous apex, an incidence of less than 0.6 cases per million population per year.6,7
Discernment of pathologic processes in this area and evaluation of their extent are critical in diagnosis and preoperative planning. Petrous apex lesions may remain undetected for extended periods because patients often complain of vague or indistinct symptoms that delay diagnosis. Headaches, atypical facial pain, mixed hearing loss, vertigo, Eustachian tube dysfunction, and middle ear effusion, while common otoneurologic complaints, may be the initial manifestations of an extensive petrous apex lesion.8
Clinical manifestations can be well explained by involvement of the structures contained within or adjacent to the apex. Compression or irritation of the Gasserian ganglion of the trigeminal nerve causes deep aural and retro-orbital pain. Diplopia results from similar compromise of the abducens nerve. Facial nerve dysfunction is less common, except in advanced lesions. Hearing loss and vestibular complaints may be caused by involvement of the eighth cranial nerve in the internal auditory canal or by direct extension of the process into the bony labyrinth.9
CASE REPORT
A 71-year-old woman presented at our outpatient department with a 1-year history of double vision when looking to the left, intermittent tinnitus, and numbness over the right cheek. She gradually became unsteady but denied having rotatory vertigo. She had undergone varicose vein surgery twice.
Examination confirmed a mild right abducent nerve palsy and hypesthesia along the distribution of the maxillary division of the right trigeminal nerve. The pure tone audiogram as well as the tympanogram were normal for the patient's age.
Magnetic resonance imaging (MRI) revealed a 3 × 2-cm homogenous mass with a high fat signal between the infrasellar skull base and the right pyramidal apex anterior to the internal auditory canal. There was evidence of slight compression of the brainstem on the ipsilateral side (Fig. 1). A subsequent computed tomography (CT) scan showed bony destruction of the right petrous pyramid up to the vestibular labyrinth. The inner ear was intact. The mass was posterior and directly adjacent to a prominent horizontal part of the ICA (Fig. 2). A provisional diagnosis of a cholesterol granuloma was made.
Figure 1.
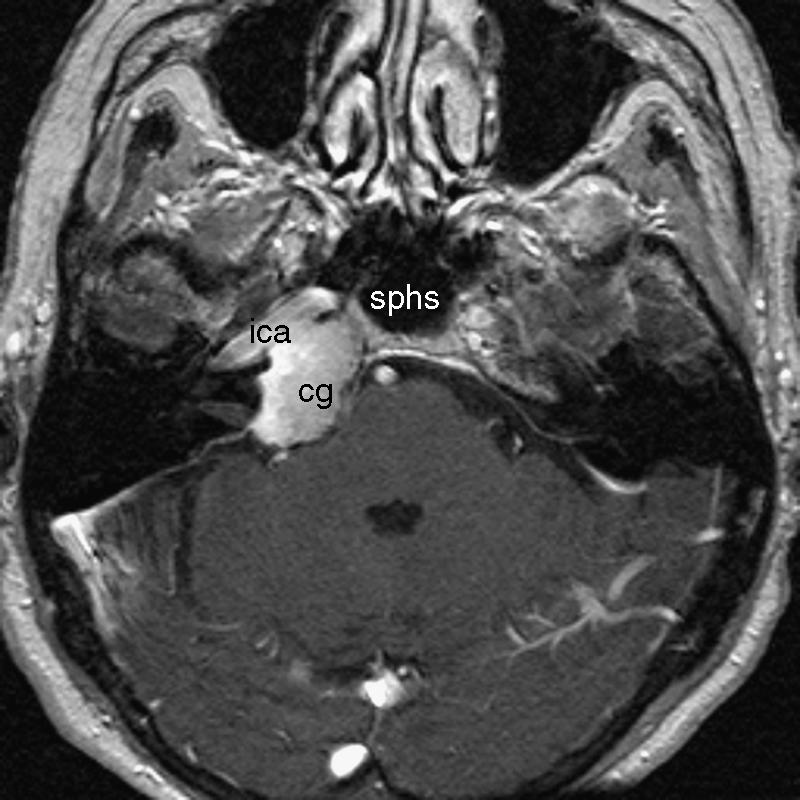
T2-weighted axial MRI shows the hypodense cholesterol granuloma (cg) involving the right petrous apex posterior to the internal carotid artery (ica). MRI, magnetic resonance imaging; sphs, sphenoid sinus.
Figure 2.
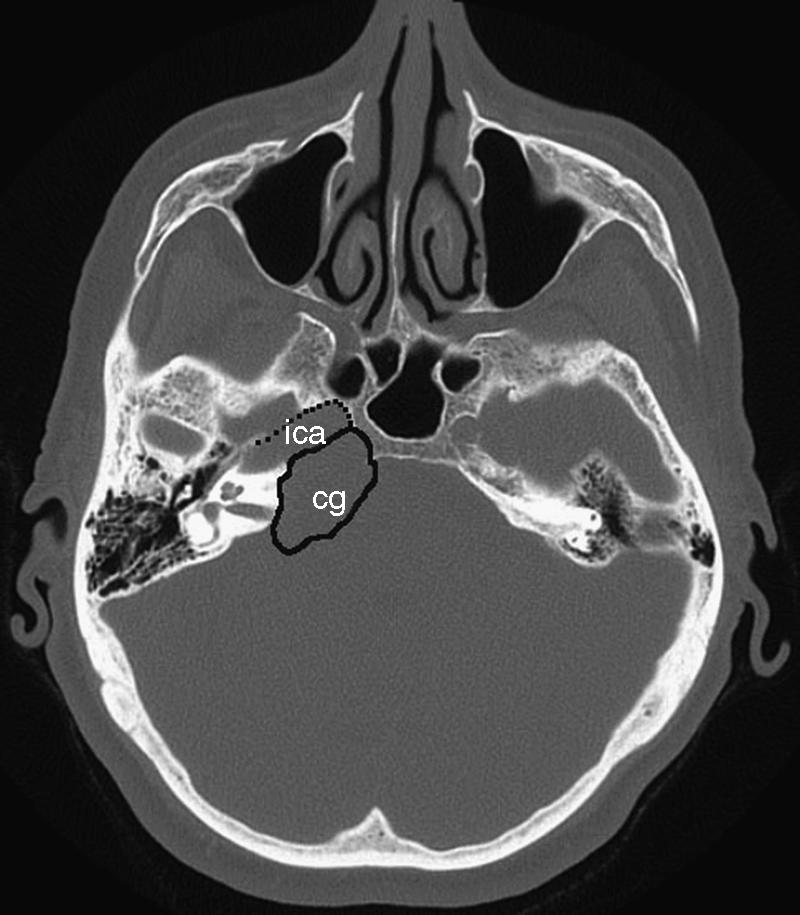
Axial CT scan showing the bony destruction caused by the cholesterol granuloma (cg) of the right petrous apex. CT, computed tomography, internal carotid artery (ica).
A transtemporal approach was performed to access and remove the lesion. A 10-cm preauricular incision extending to the parietal region was used to explore the cranial bone and to remove a 4 × 6-cm bony window placed above the root of the zygoma (two thirds of the window anterior and one third posterior to the root of the zygoma). The middle fossa dura was elevated, and the middle meningeal artery, greater superficial petrosal nerve, and ICA were identified. The lesion in the petrous pyramid was easily identified by the presence of dark material within the mass (Fig. 3).
Figure 3.
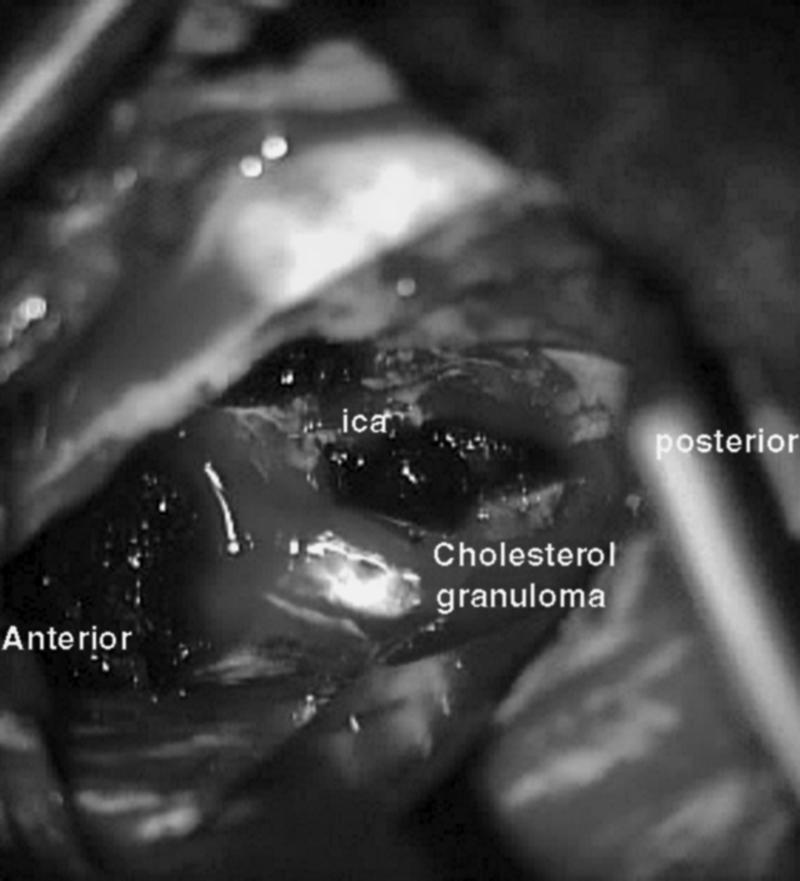
Intraoperative photograph shows the cholesterol granuloma (dark mass) exposed through the transtemporal approach. ica, internal carotid artery.
The mass was removed piecemeal under microscopic and endoscopic control using suction and cutting instruments. The resultant cavity was obliterated using abdominal fat and fascia. The patient's temporal bone graft was replaced without the need for fixation by plates. Postoperatively, the patient recovered uneventfully with improved function of the right abducent nerve. Her postoperative audiogram showed no significant change from preoperative levels.
After 6 months the T2-weighted MRI showed organized fatty tissue at the former location of the cholesterol granuloma (Fig. 4).
Figure 4.
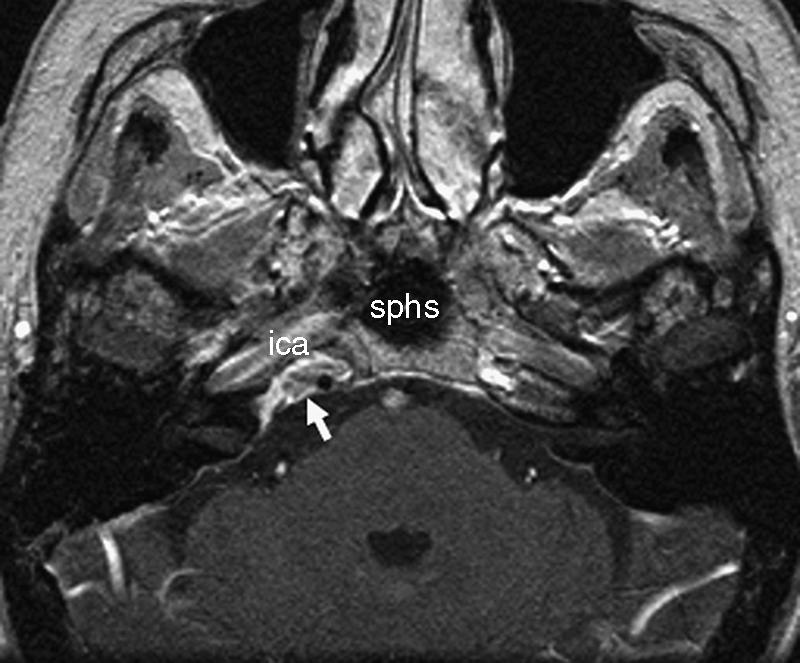
T2-weighted axial MRI obtained 6 months after surgery shows organized fatty tissue at the right petrous apex posterior to the ica (arrow). ica, internal carotid artery; sphs, sphenoid sinus.
DISCUSSION
The petrous portion of the temporal bone lies in a complicated anatomic position and has critical relationships to important neural and vascular structures. Consequently, lesions arising within or spreading to the petrous apex can result in severe clinical sequelae.
At our institution we routinely use a combination of high-resolution CT and MRI to investigate intracranial lesions. The combination of both imaging modalities has facilitated the diagnosis of different petrous apex lesions. These lesions can be classified as either cystic or solid lesions. Cholesterol granulomas are by far the most common cystic lesions found in the petrous apex.10 Cholesterol granulomas are expansile and erosive on CT, with well-defined margins. On CT, however, it is difficult to distinguish them from other lesions, such as epidermoids and mucoceles.11 In contrast, MRI shows cholesterol granulomas with greater specificity. These lesions are high intensity in T1- and T2-weighted sequences.12 Areas of interspersed low-signal intensity related to hemosiderin or granulation tissue may be enhanced with contrast media.11 Preoperatively, detailed analysis of MRI and CT scans is mandatory to define the anatomy of the jugular bulb, facial nerve, sigmoid sinus, bony labyrinth, and posterior wall of the sphenoid and ICA.
The treatment strategies for cholesterol granulomas of the petrous apex include drainage and complete excision of the capsule or procedures to drain the cyst.13 Complete surgical removal is rarely indicated because the lesion lacks an epithelial lining. Multiple surgical approaches have been described for treating these lesions. The choice of surgical approach depends on location, extension of the lesion, the anatomy of surrounding structures, and the degree and quality of hearing.6
The trans-sphenoidal approach14 provides drainage into the paranasal sinus system and preserves hearing. In our case, however, the dissection would have been severely limited laterally by the ICA. The approach also places the optic nerve at risk and has been associated with a high rate of recurrence for this lesion.15 Both the translabyrinthine16 and the transcochlear17 routes would have provided a wide exposure but would have destroyed hearing. The infralabyrinthine,18 subcochlear,19 and middle fossa20 approaches allow cochlear function to be preserved in exchange for more limited exposure and increase the risk of stricture of the drainage pathway. A high jugular bulb precludes infralabyrinthine access, and poor development of hypotympanic air cells obscures a subcochlear approach.21
In our patient we discussed the trans-sphenoidal versus the middle fossa (transtemporal) approach to access the lesion. We would have preferred the trans-sphenoidal approach so that the process could be drained. Imaging revealed only a 5 × 3-mm bony window at the posterior wall of the left sphenoidal sinus immediately adjacent to the septum intersphenoidale, which inserts in front of the ICA (Fig. 2). Therefore, to reach the cholesterol granuloma, we chose the safer transtemporal approach. Although the transtemporal approach requires a degree of temporal lobe retraction that may cause complications, including seizures in elderly patients, in our hands most patients tolerate this procedure well with minimal morbidity. Our patient made a full recovery and was ambulant within 3 days of the procedure.
Drainage of the cholesterol granuloma through the transtemporal approach into the mastoid cells may prove difficult to establish, primarily due to the difficulty encountered in placing the stent and maintaining its patency.21 We obliterated the resulting dead space after endoscopic aspiration of the granuloma and removal of the surrounding epithelium using abdominal fat to avoid the need for drainage into the mastoid cells with the potential for obstruction of the drainage pathway. MRI can readily differentiate between the fat used for obliteration and a recurrence of the cholesterol granuloma.
One criticism of the middle fossa approach is the reduced surgical exposure.14 We found that the use of endoscopy to complement the operating microscope allows adequate visualization of the petrous apex and overcomes the narrow field encountered in this approach. The developments in skull base instruments available to the neuro-otologist have facilitated the removal of lesions in this intricate anatomic location.
REFERENCES
- Chole R A. Petrous apicitis: surgical anatomy. Ann Otol Rhinol Laryngol. 1985;94:251–257. [PubMed] [Google Scholar]
- Moore K R, Harnsberger H R, Shelton C, Davidson H C. “Leave me alone” lesions of the petrous apex. AJNR Am J Neuroradiol. 1998;19:733–738. [PMC free article] [PubMed] [Google Scholar]
- Brackmann D E, Toh E H. Surgical management of petrous apex cholesterol granulomas. Otol Neurotol. 2002;23:529–533. doi: 10.1097/00129492-200207000-00023. [DOI] [PubMed] [Google Scholar]
- Nager G T, Vanderveen T S. Cholesterol granuloma involving the temporal bone. Ann Otol Rhinol Laryngol. 1976;85:204–209. doi: 10.1177/000348947608500204. [DOI] [PubMed] [Google Scholar]
- Thedinger B A, Nadol J B, Jr, Montogomery W W, Thedinger B S, Greenberg J J. Radiographic diagnosis, surgical treatment and long-term follow-up of cholesterol granulomas of the petrous apex. Laryngoscope. 1989;99:896–907. doi: 10.1288/00005537-198909000-00003. [DOI] [PubMed] [Google Scholar]
- Lo W W, Solti-Bohmann L G, Brackmann D E, Gruskin P. Cholesterol granuloma of the petrous apex: CT diagnosis. Radiology. 1984;153:705–711. doi: 10.1148/radiology.153.3.6494466. [DOI] [PubMed] [Google Scholar]
- Howitz M F, Johansen C, Tos M, Charabi S, Olsen J H. Incidence of vestibular schwannoma in Denmark 1977-1995. Am J Otol. 2000;21:690–694. [PubMed] [Google Scholar]
- Mosnier I, Cyna-Gorse F, Grayeli A B, et al. Management of cholesterol granulomas of the petrous apex based on clinical and radiological evaluation. Otol Neurotol. 2002;23:522–528. doi: 10.1097/00129492-200207000-00022. [DOI] [PubMed] [Google Scholar]
- Chole R A, Donald P J. Petrous apicitis. Clinical considerations. Ann Otol Rhinol Laryngol. 1983;92:544–551. doi: 10.1177/000348948309200603. [DOI] [PubMed] [Google Scholar]
- Larson T L. Petrous apex and cavernous sinus: anatomy and pathology. Semin Ultrasound CT MR. 1993;14:232–246. doi: 10.1016/s0887-2171(05)80083-6. [DOI] [PubMed] [Google Scholar]
- Palacios E, Valvassori G. Petrous apex lesions: cholesterol granuloma. Ear Nose Throat J. 1999;78:234. [PubMed] [Google Scholar]
- Muckle R P, Cruz A De la, Lo W M. Petrous apex lesions. Am J Otol. 1998;19:219–225. [PubMed] [Google Scholar]
- Jaramillo M, Windle-Taylor P C. Large cholesterol granuloma of the petrous apex treated via subcochlear drainage. J Laryngol Otol. 2001;115:1005–1009. doi: 10.1258/0022215011909611. [DOI] [PubMed] [Google Scholar]
- Michaelson P G, Cable B B, Mair E A. Image-guided transphenoidal drainage of a cholesterol granuloma of the petrous apex in a child. Int J Pediatr Otorhinolaryngol. 2001;57:165–169. doi: 10.1016/s0165-5876(00)00456-0. [DOI] [PubMed] [Google Scholar]
- Griffith A J, Terrell J E. Transsphenoid endoscopic management of petrous apex cholesterol granuloma. Otolaryngol Head Neck Surg. 1996;114:91–94. doi: 10.1016/S0194-59989670289-9. [DOI] [PubMed] [Google Scholar]
- Haberkamp T. Surgical anatomy of the transtemporal approach to the petrous apex. Am J Otol. 1997;18:501–516. [PubMed] [Google Scholar]
- Horn K L, Hankinson H L, Erasmus M D, Beauparalant P A. The modified transcochlear approach to the cerebellopontine angle. Otolaryngol Head Neck Surg. 1991;104:37–41. doi: 10.1177/019459989110400108. [DOI] [PubMed] [Google Scholar]
- Fong B P, Brackmann D E, Telischi F F. The long-term follow-up of drainage procedures for petrous apex cholesterol granulomas. Arch Otolaryngol Head Neck Surg. 1995;121:426–430. doi: 10.1001/archotol.1995.01890040050008. [DOI] [PubMed] [Google Scholar]
- Ghorayeb B Y, Jahrsdoerfer R A. Subcochlear approach for cholesterol granulomas of the inferior petrous apex. Otolaryngol Head Neck Surg. 1990;103:60–65. doi: 10.1177/019459989010300109. [DOI] [PubMed] [Google Scholar]
- House W F, Hitselberger W E, McElveen J T, Shelton C. In: Wilkins RH, Rengachary SS, editor. Neurosurgery Update I. New York, NY: 1990. The middle cranial fossa approach to lesions of the temporal bone and cerebellopontine angle. pp. 321–327. McGraw-Hill.
- Gianoli G J, Amedee R G. Hearing results in surgery for primary petrous apex lesions. Otolaryngol Head Neck Surg. 1994;111:250–257. doi: 10.1177/01945998941113P114. [DOI] [PubMed] [Google Scholar]


