Abstract
Dowling-Degos disease (DDD) is an autosomal dominant genodermatosis characterized by progressive and disfiguring reticulate hyperpigmentation of the flexures. We performed a genomewide linkage analysis of two German families and mapped DDD to chromosome 12q, with a total LOD score of 4.42 (θ=0.0) for marker D12S368. This region includes the keratin gene cluster, which we screened for mutations. We identified loss-of-function mutations in the keratin 5 gene (KRT5) in all affected family members and in six unrelated patients with DDD. These represent the first identified mutations that lead to haploinsufficiency in a keratin gene. The identification of loss-of-function mutations, along with the results from additional functional studies, suggest a crucial role for keratins in the organization of cell adhesion, melanosome uptake, organelle transport, and nuclear anchorage.
Dowling-Degos disease (DDD [MIM 179850]) is an autosomal dominant form of a reticulate pigmentary disorder. This rare genodermatosis was first described by Dowling and Freudenthal in 1938 and was termed dermatose reticulée des plis by Degos and Ossipowski [1954]. Further families and individual cases have since been reported (Crovato et al. 1983; Biltz and Kiessling 1988; Milde et al. 1994).
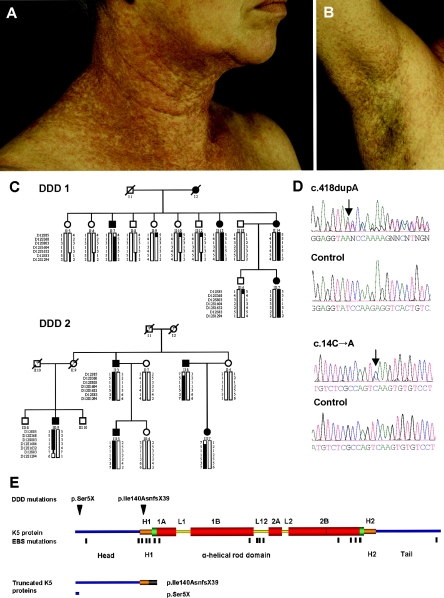
Affected individuals develop a postpubertal reticulate hyperpigmentation that is progressive and disfiguring and small hyperkeratotic dark-brown papules that mainly affect the flexures and great skin folds (fig. 1A and 1B). Pitted perioral acneiform scars (Jones and Grice 1978; Crovato et al. 1983) and genital and perianal reticulated pigmented lesions have also been described (Milde et al. 1992; O’Goshi et al. 2001; Jafari et al. 2003). Patients usually show no abnormalities of the hair or nails, though burning or itching does occur in some patients. Histology shows filiform epithelial downgrowth of epidermal rete ridges, with a concentration of melanin at the tips. No effective therapy is yet available. Differential diagnosis is sometimes complicated by the clinical overlap with other reticulate pigmentary disorders. The disorder that most closely resembles DDD is reticulate acropigmentation of Kitamura, for which location of the pigmented lesions is primarily acral, onset is within the first 2 decades of life, and palmar and plantar pits are commonly found (Griffiths 1984).
Figure 1.
Clinical appearance and underlying genetic defect of DDD. A and B, Reticulate hyperpigmentation and hyperkeratotic dark-brown papules on the neck and the axilla of individual II:13 (pedigree DDD 1). C, Two pedigrees segregating for DDD. Marker haplotypes on chromosome 12q13.11-12q15 that are linked to DDD are indicated by black bars. D, Sequence analysis showing the c.418dupA and c.14C→A mutations in patients with DDD. E, Domain structure and position of mutations in K5. Head and tail domains are marked in blue. Conserved helical end domains are marked in green, and nonhelical linker domains L1, L12, and L2 are marked with yellow bars. 1A, 1B, 2A, and 2B indicate rod subdomains. The thin red line in coil 2B indicates the “stutter.” H1 and H2 are conserved α-helical subdomains flanking the rod. The head domain extends from aa 1 to 132, H1 from 133 to 158, 1A from 159 to 203, 1B from 216 to 316, 2A from 334 to 352, 2B from 360 to 481, H2 from 481 to 501, and the tail domain from 502 to 590. The positions of the mutations p.Ser5X (c.14C→A) and p.Ile140AsnfsX39 (c.418dupA) are indicated by black arrowheads. Mutations causing EBS are marked by black lines. Detailed information about the localization of EBS mutations is available at the Human Intermediate Filament Mutation Database. The residual K5 polypeptides are generated by the p.Ser5X and p.Ile140AsnfsX39 mutations. The truncated K5 protein caused by p.Ile140AsnfsX39 encompasses positions 1–139 of K5 and 39 aa of an incorrect reading frame (gray bar).
No studies of the molecular genetic basis of DDD are, to our knowledge, available to date. Here, we describe localization of the first DDD locus on chromosome 12q and provide evidence that loss-of-function mutations in the keratin 5 gene (KRT5) lead to DDD and are suggestive of a crucial role for keratins in the organization of cell adhesion, melanosome uptake, organelle transport, and nuclear anchorage.
Methods and Results
To identify the causative gene defect, we collected blood samples from two German pedigrees described elsewhere (Biltz and Kiessling 1988; Milde et al. 1994) and composed of 24 individuals, 9 of whom were affected. Informed consent was obtained from all participants. DNA was prepared in accordance with standard methods. The detailed clinical data of these families have been presented elsewhere (Biltz and Kiessling 1988; Milde et al. 1994).
As a first step, a total genome scan was performed using 500 highly polymorphic microsatellite markers, at an average 8-cM density. Two-point LOD scores were calculated between each marker locus and DDD, with the use of LINKAGE version 5.1 software (Lathrop et al. 1984) and with the assumption of autosomal dominant inheritance, equal male and female recombination rates, a model of 95% penetrance (phenocopy rate 1%), and a disease-allele frequency of 0.0001. We found evidence of linkage to the marker D12S368, with a maximum cumulative LOD score of 4.42 (θ=0.0). Haplotypes were then reconstructed to determine the critical recombination events. A common haplotype spanning a 20.6-Mb region on chromosome 12q13.11-12q15 flanked by microsatellite markers D12S85 and D12S1294 (fig. 1C) segregated in all affected family members. A search for candidate genes in this region identified the keratin (KRT) gene cluster that contains strong candidate genes for a skin disease. We amplified by PCR and directly sequenced the region encoding the rod domains of the possible candidate genes KRT1, KRT5, KRT6A, KRT6B, and KRT6L, including the exon-intron boundaries in two patients and in one unaffected individual from our DDD-affected families. The PCR products were purified with the GFX PCR DNA Purification Kit (Amersham Biosciences) and were directly sequenced using the BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) on an ABI 3100 genetic analyzer (Applied Biosystems). In two patients, we found a single adenine base insertion in the KRT5 gene (c.418dupA; p.Ile140AsnfsX39 [GenBank accession numbers NM_000424 and NP_000415]) (figs. 1D and 1E), that led to a frameshift and that resulted in a premature termination of translation at codon 178. We observed full segregation of p.Ile140fs in the respective families. Haplotype and SNP analysis revealed that the mutation arose on the same genetic background in the two families, suggesting a common ancestor. In addition, we screened eight patients not belonging to the original pedigrees for all exons of KRT5. For three of them, no family history was known (Wenzel et al. 2002; Braun-Falco and Ring 2003). The disorder in three patients seemed to be sporadic, since parents and siblings of these patients were reported as being unaffected (Rütten and Strauß 1995; Wenzel et al. 2002); one patient had an affected father, and the last patient had an affected mother and sister (Braun-Falco et al. 2001). In five of the eight screened patients, the p.Ile140fs mutation was also identified; among them were the two familial cases (Braun-Falco et al. 2001), one patient with an unknown family history, and two patients who were reported as sporadic cases (Rütten and Strauß 1995; Wenzel et al. 2002). Genotyping of additional markers around the mutation showed a result compatible with the existence of a common ancestor for all affected individuals. However, a large number of SNPs were uninformative, and haplotypes could not be constructed for those patients for whom DNA from additional family members was not available. We also found a nonsense mutation resulting in a premature stop codon (c.14C→A; p.Ser5X) (fig. 1D and 1E) in one patient with DDD (Braun-Falco and Ring 2003). No information on family history was available for this patient. We found no mutation in KRT5 in two of the eight patients.
Neither the mutation p.Ile140fs nor p.Ser5X was detected among 530 control chromosomes of German origin. Previous studies have demonstrated a role for keratin 5 (K5) in the pathogenesis of epidermolysis bullosa simplex (EBS) (Magin et al. 2004; Omary et al. 2004). None of the individuals with DDD whom we investigated, however, showed additional skin abnormalities.
K5 and K14 form the intermediate filament (IF) cytoskeleton in basal keratinocytes of stratified epithelia and belong to the type I and type II gene families of IF proteins, which encompass 28 and 26 members in the two families, respectively (Hesse et al. 2001, 2004). In epithelia, distinct pairs of a type I and a type II protein are expressed to form an IF cytoskeleton with unique mechanical properties. They show specific interactions with desmosomal and hemidesmosomal plakins and armadillo proteins (Fontao et al. 2003; Getsios et al. 2004; Magin et al. 2004). The primary control of keratin expression occurs at the transcriptional level individually for each gene and serves to produce equal amounts of type I and type II keratin mRNAs (Coulombe and Wong 2004). All keratins consist of an extended α-helical rod domain flanked by a carboxyterminal tail and an aminoterminal head domain, the latter of which is crucial for IF formation. Assembly proceeds through formation of heterodimeric coiled coils of a type I and a type II keratin into higher-order structures. Their formation requires the head domain (Herrmann and Aebi 2004). Unpaired type I and II proteins are normally degraded but can be stabilized by being bound to associated proteins, including small chaperones and 14-3-3 proteins (Coulombe and Wong 2004). Discovery of disease mutations has revealed that the interaction of keratins with desmosomes and the nuclear envelope is crucial for the formation of a supracellular cytoskeleton, signal transduction, and the protection of the epidermis against stress (Omary et al. 2004). To date, all mutations identified in K5 have been missense mutations acting as dominant-negative mutations. Mutations at the rod-end domains lead to aggregation of the cytoskeleton and cause severe forms of EBS. Mutations in other regions of the rod domains cause milder forms of EBS, whereas those in the K5 tail domain cause migratory circinate erythema, possibly by affecting the interaction between desmosomes and keratins (Gu et al. 2003; Magin et al. 2004).
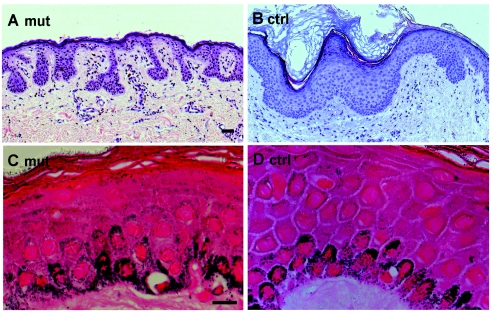
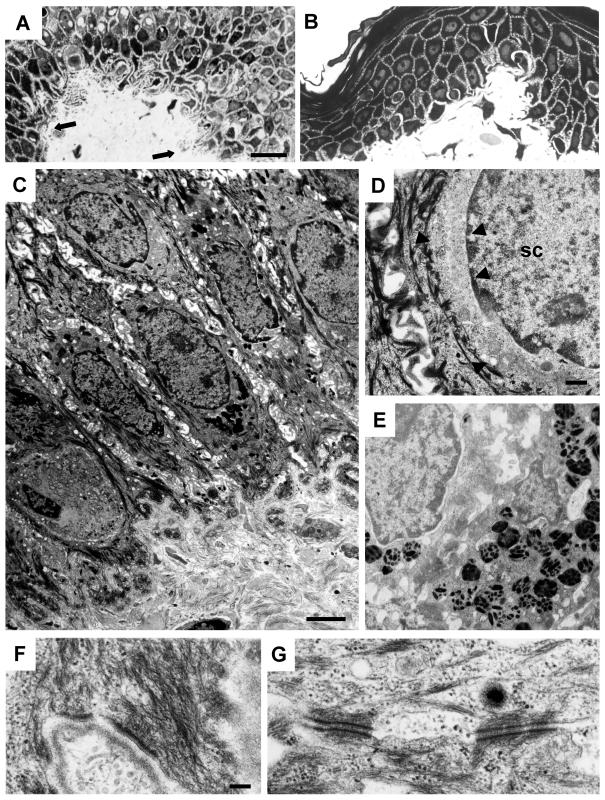
We performed further studies to understand the functional consequences and to exclude a dominant-negative effect of p.Ile140fs, which leaves most of the head domain intact. By amplifying the predicted mutant K5 mRNA by RT-PCR, we found a similar abundance of normal and mutant cDNAs, a finding that is in agreement with the prediction based on the position of the mutation (Maquat 2005). Skin biopsies from patients with p.Ile140fs and from control individuals were examined by light and electron microscopy. Histology and electron microscopy were performed as described by Reichelt et al. (2001). Fontana-Masson staining of paraffin-embedded sections was performed as described by Byers et al. (2003). Affected individuals displayed papillary downgrowth of the epidermis accompanied by filiform extensions of basal keratinocytes into the dermis (figs. 2A and 3A; for a control, see figs. 2B and 3B). Their melanosomes had a scattered distribution throughout basal keratinocytes and appeared irregularly shaped, whereas, in controls, they maintained their supranuclear caplike pattern (fig. 2C and 2D). In addition, melanosomes persisted throughout the epidermis of affected individuals, suggesting a delayed degradation (fig. 2C; for a control, see fig. 2D). Examination of semithin sections of affected skin revealed a locally altered epidermal morphology with keratinocytes of irregular shape and size. The basal aspect of mutant keratinocytes displayed long and irregular extensions into the dermis (fig. 3A), in contrast to controls (fig. 3B). Most notably, the nuclei of suprabasal but not of basal cells displayed an IF-free halo of cytoplasm with few residual organelles, suggesting an altered organization of perinuclear keratin IF (fig. 3C and 3D). The haphazard distribution of melanosomes was confirmed by ultrastructural analysis (fig. 3C). At higher resolution, it became apparent that the organization of melanin pigments in melanosomes was irregular, indicating that they represented stage III and IV organelles (fig. 3E). The overall length of keratin filaments, however, was very similar to that in control samples. Filaments displayed no tendency to aggregate or to bundle and maintained their connection to hemidesmosomes and desmosomes (fig. 3F and 3G). There was no cytolysis. The observed alterations were very similar, if not identical, to those reported elsewhere for the patient bearing the p.Ser5X mutation (Braun-Falco and Ring 2003).
Figure 2.
Light microscopy and Fontana-Masson staining of sections from DDD and control skin. A, Hematoxylin and eosin staining of sections from DDD demonstrates papillary epidermal downgrowth, in contrast to control skin (B). C, Fontana-Masson staining shows scattered distribution of melanosomes in patients with DDD. D, Regular, nuclear caplike structure of melanosomes in control skin. Scale bar represents 50 μm.
Figure 3.
Electron microscopy of skin from an individual with DDD and from a control. A, Semithin sections from a patient with DDD with filiform epithelial downgrowth (arrows), in contrast to control skin (B). Note irregular DDD cell shape and size in (A). C–G, Ultrathin sections from a patient with DDD with scattered distribution of melanosomes (E) and altered perinuclear organization of keratins in suprabasal but not in basal cells. C and D, Perinuclear rim of filament-free, smooth cytoplasm is demarcated by arrowheads; SC = spinous cell. K5 haploinsufficiency allows formation of normal keratin filaments and their interaction with hemidesmosomes (F) and desmosomes (G). Scale bars represent 20 μm (A and B), 25 μm (C), 500 nm (D and E), and 200 nm (F and G).
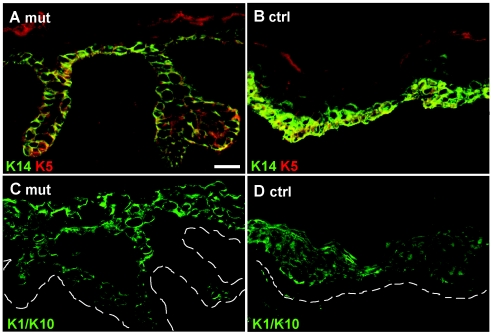
We examined keratin expression with antibodies against the K5 carboxyterminus, K14, K1, and K10. Furthermore, we produced a novel K5 head domain-specific antiserum (fig. 4A–D). This revealed no major difference between DDD and control cells. Stable transfection of MCF-7 and HaCaT cells with an EYFP-p.Ile140fs construct followed by immunofluorescence analysis showed a diffuse distribution of the mutant protein (fig. 5A). The K5 expression construct EYFP-p.Ile140fs was created as follows. A K5 cDNA was synthesized from human HaCaT keratinocytes, with amplimers 5′-AAGCTCTCGCCAGTCAAGTGTGTCC-3′ and 5′-CATTTTATTGAACACATTCTGGAGG-3′. These replaced the translation start codon with a HindIII restriction site. The cDNA was cloned into the pCR II-TOPO vector (Invitrogen). Using the QuikChange Site-Directed Mutagenesis Kit (Stratagene), we created the mutation with the forward and reverse primers 5′-GCCCTCCTGGAGGTAATCCAAGAGGTCACTG-3′ and 5′-CAGTGACCTCTTGGATTACCTCCAGGAGGGC-3′, respectively, and verified it by sequence analysis. The mutated K5 was then cloned into the HindIII/XbaI site of the pEYFP-C1 vector (BD Biosciences Clontech). The mutation leads to a frameshift in K5 at position Ile140, resulting in the following aberrant protein sequence starting at position 140: NPRGHCQPESPDSPQPANRPQHPEGEDRGARADQDPQQ.
Figure 4.
Immunofluorescence analysis of skin sections. Skin sections from a patient with DDD (A and C) and from an unaffected individual (B and D) stained with antibodies against K5 and K14 (A and B) and K10 (C and D) revealed no differences in the distribution and organization of keratins. Note the presence of terminally differentiated cells in the papillary downgrowth. The dashed lines in panels C and D indicate the basal lamina. Scale bar represents 50 μm.
Figure 5.
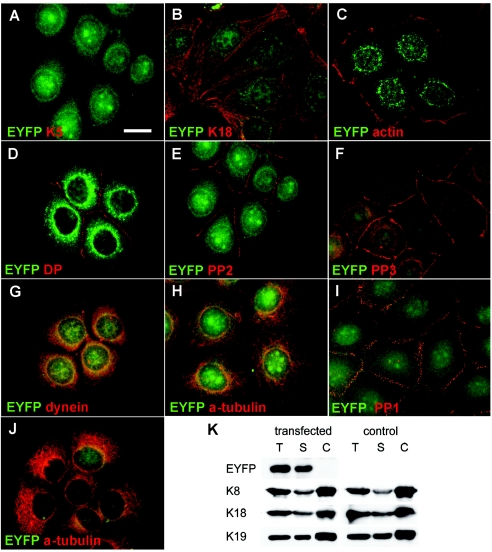
Immunofluorescence and biochemical analysis of transfected MCF-7 and HaCaT cells stably expressing an EYFP-p.Ile140fs fusion protein. A and B, MCF-7 cells showing random distribution of the K5 head domain throughout transfectants, including nuclei. Staining for K18, representative of endogenous keratins, revealed no colocalization of EYFP-p.Ile140fs with endogenous keratins (B). Stably transfected MCF-7 cells are stained with antibodies against actin (C), desmoplakin (DP) (D), plakophilin 2 (PP2) (E), and plakophilin 3 (PP3) (F). Stably transfected HaCaT keratinocytes are stained with antibodies against plakophilin 1 (PP1) (I) and α-tubulin (J). In both cell lines, no colocalization or altered distribution between EYFP-p.Ile140fs and any of these proteins was detected. In MCF-7 cells, a partial colocalization of EYFP-p.Ile140fs (yellow overlay) with dynein intermediate chain (G), but not with α-tubulin (H), was detected. Fractionation and immunoblotting of MCF-7 cells stably transfected with EYFP-p.Ile140fs and mock-transfected controls (K). Blots containing total (T), soluble (S), and Triton/high-salt–insoluble cytoskeletal proteins (C) were incubated with antibodies against EYFP, K8, K18, and K19. EYFP-p.Ile140fs was detected exclusively in the soluble fraction harvested from transfected MCF-7 cells, without affecting the solubility of endogenous keratins. Scale bar represents 20 μm.
MCF-7 cells were cultivated and transfected as described elsewhere (Werner et al. 2004). Human HaCaT keratinocytes were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum and transfected with calcium phosphate mammalian cell transfection kit (Eppendorf 5 Prime). Stably transfected cells were selected in the presence of 700 μg/ml geneticin (Invitrogen). Immunofluorescence analysis was performed as described elsewhere (Reichelt and Magin 2002). The following antibodies were used: B5-1-2 against α-tubulin (1:1,000) (Sigma), MAB1618 against dynein intermediate chain (1:50) (Chemicon), Ks18.4 against K18 (1:10) (Progen), K14 LL001 against K14 (neat) (gift from B. Lane) and K5-head against the K5-head domain (1:2,000) (Magin), Ks8.60 against K1/K10/K11 (1:200) (Sigma), ab6276 against beta actin (1:400) (abcam), 5C2 against plakophillin 1 (neat), αplakophilin 2 (neat) (gift from W. Franke), and α-plakophilin 3 (1:300) (Zymed), and Il-5F against desmoplakin (1:50) (gift from D. Garrod). Secondary antibodies were Alexa 488 or 594 conjugated goat α-mouse (1:400) and α-guinea pig (1:400) antisera (Molecular Probes). Double-immunofluorescence with antibodies to desmoplakin, plakophilins 1–3, and α-tubulin (fig. 5D–F and 5H–J) revealed no colocalization or mistargeting of these proteins under the fixation conditions used. In accordance with electron microscopy data, this excluded a role of the K5 head in desmoplakin anchorage, contrary to biochemical data reported elsewhere (Smith and Fuchs 1998). Using an antibody against dynein intermediate chain, we noted a partial colocalization of the EYFP-K5 mutant at the nuclear periphery (fig. 5G).
Fractionation of cell extracts was performed as described elsewhere (Werner et al. 2004). Total protein extracts were prepared as published elsewhere (Reichelt et al. 1999) and were subjected to SDS-PAGE. The following antibodies were used: Jl-8 against YFP (1:10,000) (BD Biosciences Clontech), Ks18.4 against K18, Ks8.7 against K8, and Ks19.2 against K19 (each 1:15,000) (Progen). Secondary antibodies were horseradish peroxidase-coupled goat α-mouse (1:30,000) antisera (Dianova). Fractionation of MCF-7 cells transfected with an EYFP-fusion protein construct into soluble and cytoskeletal proteins, followed by Western blotting, demonstrated that the p.Ile140fs polypeptide was not incorporated into the intermediate filament network but remained completely in the soluble fraction (fig. 5K). In most in vivo settings, the soluble fraction of keratins is very small, but phosphorylation of Ser residues in the head domains can lead to an increase in soluble keratins and is a prerequisite for the dynamic rearrangement of the cytoskeleton during cell differentiation, migration, and mitosis (Herrmann and Aebi 2004; Omary et al. 2004).
Discussion
The notion that the previously described KRT5 mutation p.Pro25Leu causes EBS with mottled pigmentation (Uttam et al. 1996) is suggestive of a distinct role for K5 in melanosome transport. With the exception of K14, for which a few nonsense mutations causing recessive and mild forms of EBS have been reported (e.g., Rugg et al. 1994), no nonsense mutations have been described in any other keratin genes to date. We have demonstrated for the first time that haploinsufficiency in a keratin gene causes epithelial remodeling, melanosome mistargeting, and altered perinuclear organization of IF—phenotypes otherwise described for molecules such as β-catenin, Rab27a, and nesprins (Marks and Seabra 2001; Jamora et al. 2003; Gruenbaum et al. 2005). The finding that the desmosomal cadherin desmocollin-3 is relocalized in one of our patients with DDD (Braun-Falco and Ring 2003) strongly suggests a much wider involvement of keratins in desmosome assembly and turnover. Remarkably, desmocollin misexpression alters β-catenin stability and epidermal differentiation, a mechanism which could potentially contribute to the papillary downgrowth (Hardman et al. 2005). Whereas the transport of melanosomes in melanocytes is mediated through actin- and myosin-based motor proteins, with no known involvement of IF proteins, very little is known about their distribution and turnover in keratinocytes (Marks and Seabra 2001). Haploinsufficiency of K5 as the cause of DDD strongly suggests that keratins are crucial in the organization of cell adhesion, melanosome uptake into keratinocytes, organelle transport, and nuclear anchorage. This indicates their involvement in intracellular transport, a function deemed so far to be restricted to microtubules and microfilaments.
In regard to a possible pathophysiological mechanism, we hypothesize that the K5 haploinsufficiency causes an excess of K14 that is not stabilized in heterodimeric complexes and filaments associated with K5. An excess of unpaired, soluble K14 may, therefore, be responsible for the pathology of DDD by competing with transport adapters, an analogy to soluble vimentin (Perlson et al. 2005). How could an excess of K14 interfere with melanosome uptake and localization? Recently, the keratin-related IF proteins vimentin, peripherin, and α-internexin were identified as proteins interacting directly with the δ-subunit of the adapter complex AP-3. In vimentin-deficient cells, lysosomal pH was less acidic, and vesicular zinc uptake and the surface content of AP-3 cargoes were significantly altered (Styers et al. 2004). In a similar fashion, keratins could regulate the availability and positioning of AP-3 complexes in keratinocytes. Alternatively, keratins could regulate the interaction of AP-3-dependent vesicles with motor proteins and, thereby, affect organelle transport and membrane protein transport. Of note, in addition to kinesin and dynein family members, all of which are known to bind to IF proteins, myosin V interacts with IF proteins (Rao et al. 2002; Chang and Goldman 2004). Given that myosin V mutations cause the pigmentation disorder Griscelli syndrome, alterations in keratin composition and organization could interfere with melanosome uptake in keratinocytes (Marks and Seabra 2001). Melanosome localization in keratinocytes has been suggested to involve the retrograde motor protein cytoplasmic dynein (Byers et al. 2003). Dynein is also known to play a major role in the retrograde transport of IF proteins. Conversely, the availability of dynein can be regulated by interaction with IF proteins (Chang and Goldman 2004). The importance of soluble IF proteins for long-range transport has been highlighted by the finding that soluble vimentin mediates the binding of activated mitogen-activated protein kinases to dynein via importin β (Perlson et al. 2005). K5 haploinsufficiency in DDD and the K5 p.Pro25Leu mutation in EBS with mottled pigmentation represent the only genetic entities described to date to cause a melanosome disorder in keratinocytes. Unlike Hermansky-Pudlak and Griscelli syndromes, which affect melanocytes, DDD and EBS with mottled pigmentation offer a unique approach to gain a mechanistic insight into melanosome uptake and for the analysis of vesicle transport in keratinocytes. Finally, our finding that K5 haploinsufficiency is a cause of DDD sounds a cautionary note for therapy approaches of dominant keratinopathies, including EBS, aiming at down-regulation of mutant alleles.
Acknowledgments
We thank all affected individuals and their families for participation in our study. We thank Dr. Christine Schmael, for help in preparing the manuscript, and Uschi Reuter, for her excellent technical assistance. The work was supported by grants from the Fund for Scientific Research-Flanders (Krediet aan Navorsers) and BONFOR (to R.C.B.) and by grants from the Deutsche Forschungsgemeinschaft collaborative research group, “Keratinocytes—Proliferation and Differentiation in the Epidermis” (M.M.N. and T.M.M.). M.M.N. was supported by the Alfried Krupp von Bohlen und Halbach-Stiftung. R.C.B. was supported by an Emmy Noether research grant from the Deutsche Forschungsgemeinschaft and formerly by a postdoctoral fellowship from the Fund for Scientific Research-Flanders.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for KRT5 [accession numbers NM_000424 and NP_000415])
- Human Intermediate Filament Mutation Database, http://www.interfil.org/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.gov/Omim/ (for DDD) [PubMed]
References
- Biltz H, Kiessling M (1988) Dowling-Degos disease: an autosomal dominant genetic dermatosis. Z Hautkr 63:642–644 [PubMed] [Google Scholar]
- Braun-Falco M, Ring J (2003) Enhanced cytoplasmic expression of desmocollin 3 in epidermal rete ridges of Dowling-Degos syndrome. Br J Dermatol 149:1293–1296 10.1111/j.1365-2133.2003.05645.x [DOI] [PubMed] [Google Scholar]
- Braun-Falco M, Volgger W, Borelli S, Ring J, Disch R (2001) Galli-Galli disease: an unrecognized entity or an acantholytic variant of Dowling-Degos disease? J Am Acad Dermatol 45:760–763 10.1067/mjd.2001.116340 [DOI] [PubMed] [Google Scholar]
- Byers HR, Maheshwary S, Amodeo DM, Dykstra SG (2003) Role of cytoplasmic dynein in perinuclear aggregation of phagocytosed melanosomes and supranuclear melanin cap formation in human keratinocytes. J Invest Dermatol 121:813–820 10.1046/j.1523-1747.2003.12481.x [DOI] [PubMed] [Google Scholar]
- Chang L, Goldman RD (2004) Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol 5:601–613 10.1038/nrm1438 [DOI] [PubMed] [Google Scholar]
- Coulombe PA, Wong P (2004) Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol 6:699–706 10.1038/ncb0804-699 [DOI] [PubMed] [Google Scholar]
- Crovato F, Nazzari G, Rebora A (1983) Dowling-Degos disease (reticulate pigmented anomaly of the flexures) is an autosomal dominant condition. Br J Dermatol 108:473–476 [DOI] [PubMed] [Google Scholar]
- Degos R, Ossipowski B (1954) Dermatose pigmentaire réticulée des plis. Ann Dermatol Syphiligr 81:147–151 [PubMed] [Google Scholar]
- Dowling GB, Freudenthal W (1938) Acanthosis nigricans. Br J Dermatol 50:467–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontao L, Favre B, Riou S, Geerts D, Jaunin F, Saurat JH, Green KJ, Sonnenberg A, Borradori L (2003) Interaction of the bullous pemphigoid antigen 1 (BP230) and desmoplakin with intermediate filaments is mediated by distinct sequences within their COOH terminus. Mol Biol Cell 14:1978–1992 10.1091/mbc.E02-08-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsios S, Huen AC, Green KJ (2004) Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol 5:271–281 10.1038/nrm1356 [DOI] [PubMed] [Google Scholar]
- Griffiths WA (1984) Reticulate pigmentary disorders: a review. Clin Exp Dermatol 9:439–450 [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL (2005) The nuclear lamina comes of age. Nat Rev Mol Cell Biol 6:21–31 10.1038/nrm1550 [DOI] [PubMed] [Google Scholar]
- Gu LH, Kim SC, Ichiki Y, Park J, Nagai M, Kitajima Y (2003) A usual frameshift and delayed termination codon mutation in keratin 5 causes a novel type of epidermolysis bullosa simplex with migratory circinate erythema. J Invest Dermatol 121:482–485 10.1046/j.1523-1747.2003.12424.x [DOI] [PubMed] [Google Scholar]
- Hardman MJ, Liu K, Avilion AA, Merritt A, Brennan K, Garrod DR, Byrne C (2005) Desmosomal cadherin misexpression alters beta-catenin stability and epidermal differentiation. Mol Cell Biol 25:969–978 10.1128/MCB.25.3.969-978.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H, Aebi U (2004) Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu Rev Biochem 73:749–789 10.1146/annurev.biochem.73.011303.073823 [DOI] [PubMed] [Google Scholar]
- Hesse M, Magin TM, Weber K (2001) Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J Cell Sci 114:2569–2575 [DOI] [PubMed] [Google Scholar]
- Hesse M, Zimek A, Weber K, Magin TM (2004) Comprehensive analysis of keratin gene clusters in humans and rodents. Eur J Cell Biol 83:19–26 10.1078/0171-9335-00354 [DOI] [PubMed] [Google Scholar]
- Jafari R, Tronnier M, Vakilzadeh F (2003) Morbus Dowling-Degos in genitoperianal localisation in a mother and daughter. Akt Dermatol 29:240–242 10.1055/s-2003-40570 [DOI] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E (2003) Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422:317–322 10.1038/nature01458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin TM, Reichelt J, Hatzfeld M (2004) Emerging functions: diseases and animal models reshape our view of the cytoskeleton. Exp Cell Res 301:91–102 10.1016/j.yexcr.2004.08.018 [DOI] [PubMed] [Google Scholar]
- Maquat LE (2005) Nonsense-mediated mRNA decay in mammals. J Cell Sci 118:1773–1776 10.1242/jcs.01701 [DOI] [PubMed] [Google Scholar]
- Marks MS, Seabra MC (2001) The melanosome: membrane dynamics in black and white. Nat Rev Mol Cell Biol 2:738–748 10.1038/35096009 [DOI] [PubMed] [Google Scholar]
- Milde P, Goerz G, Plewig G (1992) Dowling-Degos disease with exclusively genital manifestations. Hautarzt 43:369–372 [PubMed] [Google Scholar]
- Milde P, Süss R, Megahed M, Goerz G (1994) Morbus Dowling-Degos-Kitamura. Z Hautkr 69:282–283 [Google Scholar]
- O’Goshi K, Terui T, Tagami H (2001) Dowling-Degos disease affecting the vulva. Acta Derm Venereol 81:148 10.1080/00015550152384371 [DOI] [PubMed] [Google Scholar]
- Omary MB, Coulombe PA, McLean WH (2004) Intermediate filament proteins and their associated diseases. N Engl J Med 351:2087–2100 10.1056/NEJMra040319 [DOI] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M (2005) Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 45:715–726 10.1016/j.neuron.2005.01.023 [DOI] [PubMed] [Google Scholar]
- Rao MV, Engle LJ, Mohan PS, Yuan A, Qiu D, Cataldo A, Hassinger L, Jacobsen S, Lee VM, Andreadis A, Julien JP, Bridgman PC, Nixon RA (2002) Myosin Va binding to neurofilaments is essential for correct myosin Va distribution and transport and neurofilament density. J Cell Biol 159:279–290 10.1083/jcb.200205062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt J, Bussow H, Grund C, Magin TM (2001) Formation of a normal epidermis supported by increased stability of keratins 5 and 14 in keratin 10 null mice. Mol Biol Cell 12:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt J, Doering T, Schnetz E, Fartasch M, Sandhoff K, Magin TM (1999) Normal ultrastructure, but altered stratum corneum lipid and protein composition in a mouse model for epidermolytic hyperkeratosis. J Invest Dermatol 113:329–334 10.1046/j.1523-1747.1999.00702.x [DOI] [PubMed] [Google Scholar]
- Reichelt J, Magin TM (2002) Hyperproliferation, induction of c-Myc and 14-3-3sigma, but no cell fragility in keratin-10-null mice. J Cell Sci 115:2639–2650 [DOI] [PubMed] [Google Scholar]
- Rugg EL, McLean WH, Lane EB, Pitera R, McMillan JR, Dopping-Hepenstal PJ, Navsaria HA, Leigh IM, Eady RA (1994) A functional “knockout” of human keratin 14. Genes Dev 8:2563–2573 [DOI] [PubMed] [Google Scholar]
- Rütten A, Strauß T (1995) Morbus Galli-Galli: ein weiterer Fallbericht. Akt Dermatol 21:255–257 [Google Scholar]
- Smith EA, Fuchs E (1998) Defining the interactions between intermediate filaments and desmosomes. J Cell Biol 141:1229–1241 10.1083/jcb.141.5.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styers ML, Salazar G, Love R, Peden AA, Kowalczyk AP, Faundez V (2004) The endo-lysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol Biol Cell 15:5369–5382 10.1091/mbc.E04-03-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttam J, Hutton E, Coulombe PA, Anton-Lamprecht I, Yu QC, Gedde-Dahl T Jr, Fine JD, Fuchs E (1996) The genetic basis of epidermolysis bullosa simplex with mottled pigmentation. Proc Natl Acad Sci USA 93:9079–9084 10.1073/pnas.93.17.9079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J, Tappe K, Gerdsen R, Uerlich M, Philipp-Dormston W, Bieber T, Petrow W (2002) Successful treatment of Dowling-Degos disease with Er:YAG laser. Dermatol Surg 28:748–750 10.1046/j.1524-4725.2002.02014.x [DOI] [PubMed] [Google Scholar]
- Werner NS, Windoffer R, Strnad P, Grund C, Leube RE, Magin TM (2004) Epidermolysis bullosa simplex-type mutations alter the dynamics of the keratin cytoskeleton and reveal a contribution of actin to the transport of keratin subunits. Mol Biol Cell 15:990–1002 10.1091/mbc.E03-09-0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson Jones E, Grice K (1978) Reticulate pigmented anomaly of the flexures: Dowling Degos disease, a new genodermatosis. Arch Dermatol 114:1150–1157 10.1001/archderm.114.8.1150 [DOI] [PubMed] [Google Scholar]