Abstract
This experiment examined whether chronic stress disrupts novelty-seeking behavior under conditions that impair spatial memory. Rats were restrained for 6 h per day for 21 days, then tested in either a traditional spatial recognition Y-maze that requires extra-maze spatial cues to navigate or a version with salient intra-maze cues in addition to the extra-maze spatial cues. As previously shown, chronic restraint stress impaired performance on the spatial version of the Y-maze. However, chronically stressed rats performed well in the intra-maze cue version. The results indicate that the deficits in Y-maze performance following chronic stress are not attributed to neophobia, but likely reflect neurochemical and/or neurobiological changes underlying spatial memory ability.
Keywords: Novelty-seeking, object recognition, restraint stress, spatial memory
Introduction
Chronic stress impairs spatial memory (Conrad et al. 1996, Luine et al. 1994), but some forms of chronic stress also produce depressive-like symptoms, such as anhedonia (lack of interest in reward), anxiety and neophobia (for review, see Willner 1997). Decreased open field exploration as a result of chronic stress has been found repeatedly (Conrad et al. 2003, Conrad et al. 1999, Tejani-Butt et al. 1994). Consequently, spatial mazes that are designed to use a rat’s innate tendency to explore novelty (Granon et al. 1996) may be confounded by stress-induced changes in anxiety and novelty-seeking behavior.
One such maze, the Y-maze, is used to assess spatial recognition memory (Conrad et al. 1996). In this paradigm, the confound of reduced exploration is typically addressed by showing that chronic stress fails to affect locomotion, measured by entries into all arms (Conrad et al. 1996, 2003). At issue is that chronically stressed rats might not be motivated to seek novelty, but could still enter as many arms as controls. Therefore, making the Y-maze easier to navigate by adding intra-maze cues should help to resolve whether chronic stress reduces novelty-seeking behavior or specifically influences memory under these conditions.
In this study, chronically stressed rats were tested on one of two versions of the Y-maze: (1) the traditional version, which required rats to rely on extra-maze “spatial” cues to navigate, or (2) a version that contained salient intra-maze cues in addition to the extra-maze cues to reduce task difficulty. Chronic stress was hypothesized to influence spatial memory on the Y-maze without altering novelty-seeking behavior. Therefore, chronically stressed rats were predicted to exhibit impaired performance in the traditional Y-maze and intact performance in the Y-maze with internal cues.
Methods
The Institutional Animal Care and Use Committee at Arizona State University approved all procedures, which were in accordance with the applicable portions of the Animal Welfare Act and the “Guide for the Care and Use of Laboratory Animals” by DHHS. Thirty-two male Sprague–Dawley rats (300–400 g) were pair-housed and kept on a 12 h:12 h light/dark cycle (lights on at 19.00 h). Rats were allowed at least one week to adapt to housing conditions prior to any experimental manipulation and were given food and water ad libitum.
Rats were randomly divided into control (n = 15) and stressed (n = 17) groups, which were housed in separate sound- and temperature-controlled chambers (temp = 18–21°C). Rats in the stress group were placed in wire mesh restrainers (16 cm circumference, 24 cm length) in their home cages for 6 h a day beginning at 10:30 and ending at 16:30 for 21 consecutive days as previously reported (Luine et al. 1996, Magariños et al. 1995). This treatment allowed restraint to occur during the dark cycle to avoid disruption of sleep yet still allowed time during the dark cycle to eat and drink before and after restraint. Control and stressed rats were weighed on days 1, 7, 14 and 21 from the start of restraint.
Two versions of a spatial recognition Y-maze were used. The traditional version of the Y-maze (Trad) was similar to that originally described (Dellu et al. 1992) and has been validated for use as a test of spatial memory (Conrad et al. 1996). A second version of the Y-maze was created by including salient intra-maze cues (Cue). In this cued version, one large enamel-painted rock (approximately 400 g) was placed at the end of each arm. These rocks could be distinguished by texture (rough vs smooth) as well as paint scheme (stripes, triangles or circles). The rocks were present during training and testing.
During training, a rat was placed in the Start arm and allowed to explore the Start and Other arms for 15 min while the Novel arm was blocked with black Plexiglas. Corncob bedding on the floor of the maze was mixed before training and testing each rat, and the maze was rotated between training and testing to prevent the use of odour cues in maze navigation. The terms Novel, Start and Other refer to the spatial location of the arms with respect to extra-maze cues. Additionally, rocks at the ends of the arms remained in the same spatial location with respect to the extra-maze cues for training and testing. After a 4h inter-trial interval, the rat was placed into the Start location for testing and was allowed to explore all three arms for 5 min. The location of the Start, Novel and Other arms was counterbalanced among rats and the experimenter was never present while the rats explored the maze. All trials were videotaped and analyzed later by an experimenter who was blind to treatment condition. If memory and novelty-seeking behavior were intact, then rats were expected to enter the Novel arm more than Other arm, because of the innate tendency of rats to explore novelty (Granon et al. 1996). Training and testing occurred at least 4 h after the end of chronic restraint when corticosterone levels were no longer elevated due to the restraint paradigm.
Wilcoxon non-parametric tests were used to determine whether rats preferred the Novel arm to the Other arm. The Wilcoxon analysis compared percent of time spent (Dwell) and percent of entries (Entry) into the Novel and Other arms for each experimental group. An entry was scored when a rat’s front paws crossed into an arm. The first three minutes of exploration during testing were analyzed because rats habituate quickly to the Y-maze (Dellu et al. 1992). The Start arm was not included in the analysis because the rats were placed there at the beginning of the testing trial and it was therefore inherently biased and not orthogonal to the Novel and Other arms. Differences between groups in total entries, latency to leave the start arm and body weight were analyzed with analysis of variance (ANOVA) and significant effects were further investigated with Neuman-Keuls post hoc analyses.
Results
Chronically stressed rats demonstrated intact novelty-seeking behavior and impaired spatial memory. The Wilcoxon analysis revealed that chronically stressed rats spent more time in the Novel than the Other arm when tested on the cued maze ( p < 0.05), but spent similar amounts of time in the Novel and Other arms when tested on the traditional maze. Control rats tested on either version of the Y-maze spent more time in the Novel arm than the Other arm ( p < 0.01, Figure 1A). The entry data are consistent with these findings (Figure 1B)
Figure 1.
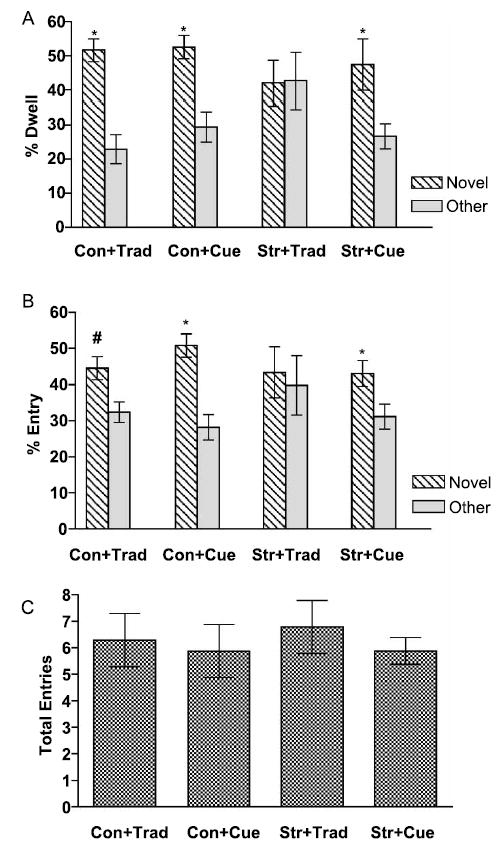
Effect of Chronic Stress on Memory and Exploration. (A) Y-maze performance based upon time spent in arms (Dwell). Rats tested on the intra-maze cue version of the Y-maze (Cue) spent more time in the Novel arm than the Other arm regardless of treatment (Con-Cue, Str-Cue). Control rats tested in the traditional Y-maze spent more time in the Novel arm more compared to the Other arm (Con-Trad), while chronically stressed rats spent similar amounts of time in the novel and other arms (Str-Trad). (B) Y-maze performance based upon entries made into arms (Entry). Rats tested on the intra-maze cue version of the Y-maze (Cue) entered the Novel arm more than the Other arm regardless of treatment (Con-Cue, Str-Cue). Control rats tested in the traditional Y-maze showed a tendency to enter the Novel arm more compared to the Other arm (Con-Trad), while chronically stressed rats entered the Novel and Other arms similarly (Str-Trad). (C) Total number of arm entries. There were no differences between groups. Trad = traditional Y-maze, Cue = intra-maze cue Y-maze, Con = control, Str = chronic stress. Rats per group were: Con + Trad n = 7, Con + Cue n = 8, Str + Trad n = 9, Str + Cue n = 8. Data are represented as means ± SEM. * p < 0.05, # p = 0.06, Novel arm compared to Other arm.
To determine whether motivation to explore the Y-maze differed between groups during testing, total entries (sum of entries into Novel, Start and Other arms) and latency to leave the Start arm were analyzed using a 2 ×2 ANOVA. Neither total entries nor latency to leave the Start arm were affected by chronic stress. Analysis of total entries throughout minutes 1–3 of testing revealed no significant main effects for stress, F(1, 28) = 0.91, p = 0.34, maze type, F(1, 28) = 0.66, p = 0.42, or their interaction, F(1, 28) = 0.02, p = 0.86 (Figure 1C). The analysis for latency to leave the Start arm revealed similar results, whereby no significant main effects were revealed for stress, F(1, 27) = 0.94, p = 0.34, maze type, F(1, 28) = 0.46, p = 0.83, or their interaction F(1, 28) = 1.46, p = 0.23; means ± SEM were as follows: Control + Trad 24.6s ± 10.2, n = 7; Control + Cue 16.6s ± 7.8, n = 8; Stress + Trad 20.0s ± 6.6, n = 9; Stress + Cue 28.8s ±10.6, n = 8.
Body weight gain was analyzed because previous studies have shown that chronic stress reduces weight gain in male rats (Conrad et al. 2001, Magariños and McEwen 1995, Watanabe et al. 1992). A 2 ×4 repeated measures ANOVA for stress treatment (control & stress) and day (1, 7, 14 & 21) revealed a significant main effect for stress, F(1, 32) = 35.49, p < 0.001, a significant main effect for day, F(3, 96) = 746.86, p < 0.001, and a significant interaction between stress and day, F(3, 96) = 141.20, p < 0.001, showing that stressed rats gained weight more slowly than controls over three weeks of restraint (Control d1 329.6 ± 4.29, d21 449.2 ± 5.49; Stress d1 342.6 ± 3.29, d21 385.5 ± 3.5).
Discussion
The hypothesis that chronic stress leaves novelty-seeking behavior intact while impairing spatial memory was supported. If the chronically stressed rats exhibited reduced novelty-seeking compared to controls, then they would have avoided the Novel arm or explored the Novel and Other arms similarly in both versions of the Y-maze. However, chronically stressed rats explored the Novel arm more than the Other arm in the intra-maze cue version of the Y-maze, but explored the Novel and Other arms similarly in the traditional version. Thus, chronic stress left novelty-seeking intact.
Several control measures strengthened the interpretation that chronic stress impaired Y-maze performance without altering novelty-seeking. Chronic restraint was verified as a stressor because chronically stressed rats gained weight more slowly than controls. Navigation by use of intramaze odors was prevented by mixing the bedding on the floor of the maze and rotating the maze between training and testing. Total entries into all arms showed that chronic stress failed to alter general exploration in the Y-maze. Therefore differences in locomotor activity failed to influence novel arm exploration. This latter outcome contrasted with other reports, which found that chronically stressed rats failed to explore as readily as control rats in the open field (Conrad et al. 2003, Conrad et al. 1999, Tejani-Butt et al. 1994) and elevated plus maze (Wood et al. 2004). One reason that chronically stressed rats in the present study explored as much as controls could be that they were in a somewhat familiar environment whereby they had explored mostof the mazeon a previoustrial. The Y-maze also had arms with high walls, unlike the center of the open field or the open arms in the elevated plus maze, allowing the rats to satisfy their innate tendency toward thigmotaxia, maintaining close proximity to walls. Overall, the Y-maze was designed to reduce anxiety (Conrad et al. 1996) while other measures that have shown reductions in exploration as a result of chronic stress were designed to be anxiogenic (Pellow et al. 1985).
Another group that specifically investigated object recognition in chronically stressed rats found different results from those presented here. Beck and Luine (1999) found that chronically stressed rats exhibited functional object recognition at short delays (up to 1-h), but were impaired at a long delay (4-h), which appears to contradict the current finding that chronically stressed rats showed intact recognition in the Y-maze at a 4-h delay. An important difference between the two studies was that the test used by Beck and Luine (1999) relied on object recognition by the rats, whereas the present study sought to simply make the Y-maze easier to navigate by including both intra- (object recognition) and extra-maze (spatial) cues. Therefore, the combination of intra- and extra-maze cues in the present study may have allowed rats to use multiple memory systems, making intact recognition memory possible at a 4-h delay.
The present data are consistent with the interpretation that chronic stress impairs performance on spatial memory tasks by altering neural systems involved in spatial memory rather than by causing neophobia. The hippocampus, a brain area long associated with spatial memory (for review, see O’Keefe et al. 1978), is likely to be involved in the memory deficits found in the present study because chronic stress produces morphological changes in the hippocampus. Specifically, chronic restraint causes retraction of apical dendrites in the CA3 region of the hippocampus (Conrad et al. 1999, Magariños and McEwen 1995, Watanabe et al. 1992). These morphological changes may make the hippocampus vulnerable to damage by metabolic challenges (Conrad et al. 2004), and prolonged social stress can lead to cell death (Uno et al. 1989). These chronic stress-induced morphological changes also lead to deficits on spatial mazes that require hippocampal function. Three weeks of daily restraint impairs spatial memory in male rats tested on the eight-arm radial maze (Luine et al. 1994) and the Y-maze (Conrad et al. 1996, Conrad et al. 2003). Randomized housing conditions combined with cat exposure for five weeks hinders performance on a radial arm water maze (Park et al. 2001). Twelve weeks of daily cold water immersion also leads to spatial memory impairment on the eight arm radial maze (Nishimura et al. 1999). The wide range of stressors and measures of spatial memory clearly suggest that chronic stress is detrimental to spatial memory. The present data add to this literature showing that chronic stress does not mediate performance deficits on spatial memory tasks by reducing novelty-seeking behavior.
Acknowledgments
This work was funded by MH64727 (Conrad), a research incentive award from ASU College of Liberal Arts and Sciences (Conrad). We would like to thank Sarah Baran, Jamie Jackson, Lisa Wise and Rudy Bellani for their contributions. I also thank Janet Neisewander, Eddie Castañeda and Mark Reilly.
References
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: Role of monoamines and amino acids. Btrain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobio Learn Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson J, Wise L. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004;125:759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Mauldin-Jourdain M, Hobbs RJ. Metyrapone reveals that previous chronic stress differerentially impairs hippocampal dependent memory. Stress. 2001;4:305–318. doi: 10.3109/10253890109014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 1992;588:132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- Granon S, Save E, Buhot MC, Poucet B. Effortful information processing in a spontaneous spatial situation by rats with medial prefrontal lesions. Behav Brain Res. 1996;78:147–154. doi: 10.1016/0166-4328(95)00242-1. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magarinos AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physio Beh. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Nishimura J, Endo Y, Kimura F. A long-term stress exposure impairs maze learning performance in rats. Neurosci Lett. 1999;273:125–128. doi: 10.1016/s0304-3940(99)00645-x. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. 1978. The hippocampus as a cognitive map.Oxford: Clarendon Press.
- Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biol Psychia. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Meth. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Pare WP, Yang J. Effect of repeated novel stressors on depressive behavior and brain norepinephrine receptor system in Sprague-Dawley and Wistar Kyoto (WKY) rats. Brain Res. 1994;649:27–35. doi: 10.1016/0006-8993(94)91045-6. [DOI] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: Prevention by lithium treatment. Proc Natl Acad Sci USA. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]


