Abstract
Emerging data report sex differences in how the brain responds to chronic stress. Here, we investigated the effects of chronic restraint stress (6 h/day/21 days) on hippocampal morphology and function in ovariectomized female rats. Chronic restraint stress caused CA3 apical dendritic retraction in short- and long-shafted neurons, while it reduced basal dendritic arbors in long-shafted neurons only. Chronic restraint did not affect CA1 dendritic arborization, although it increased the proportion of CA1 spine heads compared with controls. Both stressed and control animals performed well on the Y-maze, a spatial memory task. However, chronic stress enhanced Y-maze performance compared with controls, which may reflect facilitated spatial memory or reduced habituation. Y-maze performance correlated with CA1 spine head proportion. This relationship suggests that spatial ability in females may be more tightly coupled with CA1 morphology, which may override the influence of CA3 dendritic retraction. Thus, this research provides additional evidence that CA3 morphology does not always parallel spatial memory.
Keywords: hippocampus, chronic stress, females, spatial recognition memory, dendritic spines
Abbreviations: ANOVA, analysis of variance; CORT, corticosterone; LS, long-shafted neurons; LTP, long-term potentiation; OVX, ovariectomy/ovariectomized; SS, short-shafted neurons
Sexual dimorphisms in the stress response have been identified for some time, and a growing literature shows that stress impacts hippocampal cognitive ability differently in the two sexes. In humans, stressed males, but not females, exhibit elevated glucocorticoid levels (Kirschbaum et al., 1992), which negatively correlate with hippocampal-dependent declarative memory (Wolf et al., 2001). Similarly in animal models, acute restraint stress impairs hippocampal-dependent spatial memory in male rats, but facilitates spatial memory in female rats (Conrad et al., 2004a). These reports indicate that understanding the stress response and cognitive ability in males may not extrapolate to females. However, few studies use females to investigate the influence of stress on brain morphology and cognitive ability, which is alarming given statistics on mental health in humans. Women with a history of long-term depression have a 10–15% reduction in hippocampal volume compared with controls (Sheline, 1996; Sheline et al., 1999) and are almost twice as likely as men to be diagnosed with depression (Heller, 1993; Kessler et al., 1993; Weissman et al., 1993). In animal models, depressive-like symptoms are often preceded by chronic stress or stressful life events, and females cope differently with stressful situations than males (Westenbroek et al., 2003). Although recent research has begun to focus on such gender differences, care and treatment of women has been derived predominantly from research on males. Therefore, more research on females is needed to investigate stress effects on the brain and body, and to better address women’s health.
In the brain, stress produces sexually dimorphic responses in both hippocampal morphology and function. In male rats, restraint for 6 h/day/21 days retracts hippocampal dendritic arbors in the CA3 region (Watanabe et al., 1992c; Magarinos and McEwen, 1995a; Magarinos et al., 1998; Conrad et al., 1999), which corresponds to impaired hippocampal-dependent spatial memory (Luine et al., 1994a,b; Conrad et al., 1996). Pharmacological blockade of CA3 dendritic retraction also correlates with the prevention of spatial memory deficits in separate studies (Watanabe et al., 1992a,b; Luine et al., 1994a,b; Conrad et al., 1996). In contrast, chronically-stressed, gonadally-intact females have less robust dendritic retraction in basal CA3 dendrites compared with the apical CA3 dendritic retraction seen in males (Galea et al., 1997). In addition, spatial memory appears unaffected or improved by chronic stress in females (Bowman et al., 2001, 2003; Conrad et al., 2003; Kitraki et al., 2004). Together, these data suggest that females can have intact or even facilitated spatial memory despite the possible presence of hippocampal dendritic retraction.
Ovarian hormones may modulate the effects of stress on hippocampal morphology and function in females. Estrogen levels have been found to induce changes in both CA1 spine density and spine shape in rodents (Gould et al., 1990; Woolley et al., 1997; Li et al., 2004; Gonzalez-Burgos et al., 2005). In addition, ovarian hormones and stress appear to have interactive effects on behavior. After 21 days of stress, female rats in proestrus show impaired spatial memory compared with rats in other stages of the estrous cycle; however, after 28 days of stress females in proestrus show improved spatial memory compared with their control counterparts (Bowman et al., 2001). Through-out the estrous cycle, the amount of corticosterone (CORT) released in response to stress differs, with greater peaks in proestrous rats (Viau and Meaney, 1991; Conrad et al., 2004a). Fluctuations in estradiol and prolactin can also stimulate CORT secretion (Lo and Wang, 2003). Therefore, these changes may modulate stress effects on the brain and subsequent behaviors. The interactions among stress, ovarian hormones, and hippocampal morphology and function are complex; thus, we designed an experiment using ovariectomized (OVX) female rats and acute estrogen replacement. This study is the first study to examine the influence of chronic restraint stress and acute estrogen treatment in OVX female rats on both hippocampal morphology (CA3 and CA1 regions) and hippocampal-dependent spatial recognition memory.
EXPERIMENTAL PROCEDURES
Subjects
The Arizona State University Institutional Animal Care and Use Committee approved all subjects and procedures used for this research. All efforts were made to minimize the number of animals per group and any potential suffering of these subjects. Two cohorts initially totaling 80 female Sprague–Dawley rats (CD strain), weighing approximately 230 g, were purchased from Charles River Laboratories (Hartford, CT, USA). All rats were randomly assigned to either a control (n = 40) or chronically-stressed group (n=40). In addition, rats were administered either 17-β estradiol or sesame oil, giving rise to four experimental conditions: control/vehicle (CV), control/estradiol (CE), stressed/vehicle, (SV) stressed/estradiol (SE). Control and stressed rats were housed in separate, but identical chambers. Housing chambers were on a 12-h light/dark schedule (lights on at 7:00 AM) with a temperature of 21–22 °C. Rats were housed two per cage with access to food and water ad libitum.
OVX
One week after arrival, rats received bilateral ovariectomies. Rats were anesthetized using a ketamine cocktail (10 ml ketamine, 5 ml xylazine, 2 ml acepromazine, 3 ml 0.9% NaCl, 1 ml/kg, i.m.). The abdomen was rubbed with Betadine surgical scrub (povidone-iodine 7.5%) and a longitudinal, 1 cm incision was made to remove the ovaries, which were tied off and then cut from the oviduct. The muscle was sutured with coated Vicryl suture thread (Ethicon, Inc., Somerville, NJ, USA), and the skin was secured with wound clips, which were removed one week post-surgery.
17-β Estradiol administration
Half of the control and stressed rats received injections (0.1 mL, s.c.) of either 5 μg 17-β estradiol (Steraloids, Inc., Newport, RI, USA) or sesame oil. Injections were given to each rat at 48 h and then again at 24 h prior to behavioral training on the Y-maze, a schedule designed to parallel estrogen levels similar to those in proestrus (Chesler and Juraska, 2000; Korol and Kolo, 2002). The 4 week delay between OVX and estrogen treatment is within the 2–8 week window that acute estrogen replacement has been previously shown to effectively influence behavior (Becker et al., 1987; Frye, 1995; Korol and Kolo, 2002; Markham et al., 2002). Injections coincided with the last two days of restraint, and were administered to the animals between 8 and 9 AM before restraint started.
Restraint and handling
Beginning 10 days after surgery, half of the rats underwent chronic restraint stress for 6 h/day (from approximately 9 AM to 3 PM) for 21 days. Rats were restrained in their home cages in wire mesh restrainers (18 cm circumference × 24 cm long) and were transferred to larger restrainers (23 cm circumference × 28 cm long) when they outgrew the smaller restraint. Control rats remained undisturbed during the time of restraint stress and were housed in a separate but identical chamber.
To reduce potential exploration anxiety during the day of testing, rats were handled for 2 weeks, beginning on day 7 of restraint. All rats were held for one minute, allowed to explore in a large box for approximately two–three minutes, and then were held again for one minute before being returned to their cage. Handling occurred in two different rooms, both separate from the Y-maze room which was used for behavior assessment. To reduce any stress associated with the experimenter, handling was not performed by researchers who assisted with restraint or within two hours after the restraint session.
Y-maze testing
The Y-maze was used for behavioral testing of spatial recognition memory. The apparatus consisted of three identical arms (50 l×16 w×32 h cm) made of black Plexiglas. The three arms were randomly designated as the “start,” “novel,” and “other” arm and were counterbalanced between rats. Animal bedding covered the floor of the maze and was mixed between trials to prevent rats from using odor cues. Visual cues, in a variety of colors and shapes, were located outside of the maze.
The first behavioral trial took place one day after restraint stress termination and two hours after the change in light cycle. Rats were placed in the start arm and allowed to explore the start and other arms for 15 min, while a black Plexiglas divider blocked the designated novel arm. Four hours later, rats underwent testing in which they were allowed to explore all arms for 5 min. The experimenter was not visible to the rats during testing; behavior was videotaped and then quantified at a later date. During quantification, the researcher was blind to the arm assignments and the experimental conditions. Entries were defined as entrance of front paws into an arm.
Golgi procedure
Rats were injected with a lethal dose of Nembutal (1 ml, i.p.), and decapitated, then unperfused brains were rapidly removed. FD Rapid Golgistain™ kits (FD NeuroTechnologies, Baltimore, MD, USA) were used for Golgi staining, and staining was successful for one cohort of rats. Brains were prepared as directed by the kit and then quickly frozen in 2-methylbutane and cut in 100 μm sections (Microtome HM 500 OM Cryostat, −30/32 °C). Once placed on a slide, brains were firmly pressed by hand using Bibulous blotting paper (Fisher Scientific International Inc., USA). Brains were processed, left in the dark to dry for 1–2 weeks, stained according to the Golgistain™ Kit, and then coverslipped with Permount Mounting Media (Fisher Scientific International Inc.).
Histology
For proper Golgi analysis, cells were chosen based on the following criteria: 1) the cell body and dendrites were fully impregnated, 2) the cell was relatively isolated from surrounding neurons, and 3) the cell was located in either the CA3c or CA1 region of the hippocampus. A camera lucida drawing tube attached to an Olympus BX51 microscope was used to trace all neurons (320×). Dendritic length was quantified using a Scion Image Microcomputer Imaging Device Program (Scion Corporation, Frederick, MD, USA) attached to an Olympus BX 50 microscope. Branch points of the dendritic tree were also counted. The neurons used for analyses were randomly selected with the only restriction being that sample sizes were equal between the groups.
CA3
Six rats from each experimental group (total of 24 rats) were chosen in which sufficient numbers of Golgi-impregnated cells could be identified. CA3 neurons were further labeled as short-shaft (SS) or long-shaft (LS) depending on their relative location in the stratum pyramidale and proximal apical shaft length (Fitch et al., 1989). Dendritic length and branch points were measured separately for the apical and basal sections of the neuron. For each rat, apical analyses included two SS and two LS neurons averaged to obtain one value for SS and LS cells, respectively. Basal analyses were performed similarly, with three SS and three LS cells separately averaged for each rat.
CA1
A total of five rats from each group (total of 20 rats) were chosen in which sufficient Golgi staining was present. For each rat, six neurons were analyzed. CA1 cells also express two neuronal types; however, cell complexity does not differ like CA3 cells (Bannister and Larkman, 1995). Therefore, both cell types found in the CA1 region were analyzed together and averaged to obtain one measure of branch points and one measure of branch length per rat.
CA1 spine densities from pyramidal cells that had consistent Golgi impregnation were also analyzed because spines fluctuate across the estrous cycle (Woolley et al., 1990; Shors et al., 2001) and estrogen is one of the most robust modulators of spine density in the CA1 region (Shors et al., 2001). Three cells each from five rats in each group (total of 20 rats) were chosen randomly for spine analyses. Following previously established protocols (Gould et al., 1990; Woolley et al., 1997), spines from the most lateral apical tertiary dendrite and the most lateral basal secondary dendrite were quantified. A camera lucida drawing tube was used to trace spines that could clearly be seen in one focal field (1250×). Spines were then counted, and the density was calculated as the number of spines/10 μm of dendrite. There were visible differences between CA1 spine shapes. In general, young hippocampal spines are headless, and exhibit heads as they mature, usually after 2–4 weeks in vitro (Papa et al., 1995). Therefore, spines with heads were differentiated from those without heads, and we separately compared the proportion of each with total dendritic spines.
Statistical analyses
Three rats were removed due to surgical complications or equipment errors, and the final analyses for the Y-maze included 77 rats (control n = 39, stress n = 38).
Golgi
An analysis of variance (ANOVA) with treatment (control, stress) and hormone (vehicle, estradiol) as the independent variables was used to analyze the effects on CA3 and CA1 morphology. Separate comparisons were made for apical and basal branch points and length. For CA3 analyses, a within-subject design for cell type (SS, LS) was employed. For CA1 analyses, an ANOVA with spine density as the dependent variable was computed. Since CA1 dendritic spines were represented as ratios of dendritic spines with heads to total dendritic spines, the spine density data were transformed according to the equation for proportions, 2arcsin (square root (Y)) (Cohen et al., 2003).
Y-maze
To assess spatial memory within a given condition, a Wilcoxon non-parametric matched pairs test was used to compare entries into the novel and other arms. The start arm was not included in this analyses because rats were initially placed there by the experimenter, potentially creating a bias against re-entering that arm. Differences in spatial memory across groups were determined by ANOVA using treatment (control, stress) and hormone (vehicle, estradiol) as the independent variables. The dependent variable was a difference score, generated by subtracting the percentage of entries into the other arm from the percentage of entries into the novel arm. Positive scores reflect a preference for the novel arm, while negative scores indicate a preference for the other arm. A zero score indicates chance performance with no arm preference. Total entries (sum of entries into all three arms) were also used to determine whether motor or motivational factors differed among the groups (Conrad et al., 1996).
To assess the relationship between morphology and behavior, Spearman correlations (one-tail, P<0.05) were performed to compare CA3 dendritic properties or CA1 spines with total entries or difference scores.
Body weights
An ANOVA with treatment (control, stress) and hormone (vehicle, estradiol) was the between-subjects variable and day (1, 7, 14, and 21) as the within-subject variable was used to determine the effects of stress or estrogen on body weight.
Software
The statistical software program STATISTICA (Statsoft Inc., Tulsa, OK, USA) was used for all ANOVAs and Pearson correlations. Significant effects from ANOVA (P<0.05) were further analyzed using Newman-Keuls post hoc tests, unless otherwise noted. Spearman correlations were conducted using SPSS (SPSS Inc., Chicago, IL, USA, version 12.0).
RESULTS
Estrogen treatment failed to produce statistically significant effects on morphology (P>0.10), behavior (P>0.10), or weight (P>0.1). Therefore, the data were collapsed across vehicle- and estrogen-treated groups, and only analyses related to stress effects are reported. The final number of rats per group was the following for morphology: CA3 (control n = 12, stress n = 12), CA1 (control n = 10, stress n = 10), CA1 spines (control n = 10, stress n = 10).
Effects of chronic stress on hippocampal morphology
CA3 apical dendritic arborization
Chronically-stressed rats displayed apical CA3 dendritic retraction (Fig. 1). A 2 × 2 mixed-factor ANOVA (treatment × cell type) revealed a significant main effect of treatment for apical branch points, F(1,22) = 11.40, P<0.01, and total dendritic branch length, F(1,22) = 5.22, P<0.05. Restrained rats expressed fewer branch points and less total branch length than controls (Fig. 2A, B). As expected, a significant effect of cell type was also demonstrated, with SS cells having more branch points, F(1,22) = 9.35, P<0.01, and greater overall branch length, F(1,22) = 4.47, P<0.05, compared with LS cells, regardless of treatment. All other effects were not significant (P>0.10). Pearson correlations failed to demonstrate any significant relationships between CA3 apical branch points and length and difference scores, P>0.10
Fig. 1.
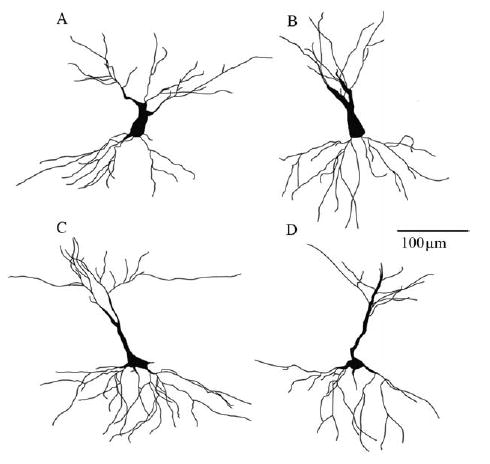
Golgi-Stained CA3 Neurons. Camera lucida drawings (320×) represent experimental conditions of the two CA3 neuronal types studied. (A) Control SS, (B) Stress SS, (C) Control LS, and (D) Stress LS. Note the reduction in apical dendritic complexity in the stressed (B, D) vs. control neurons (A, C). Basal dendritic retraction was evident in the LS stressed group only (D) compared with LS control neurons (C).
Fig. 2.
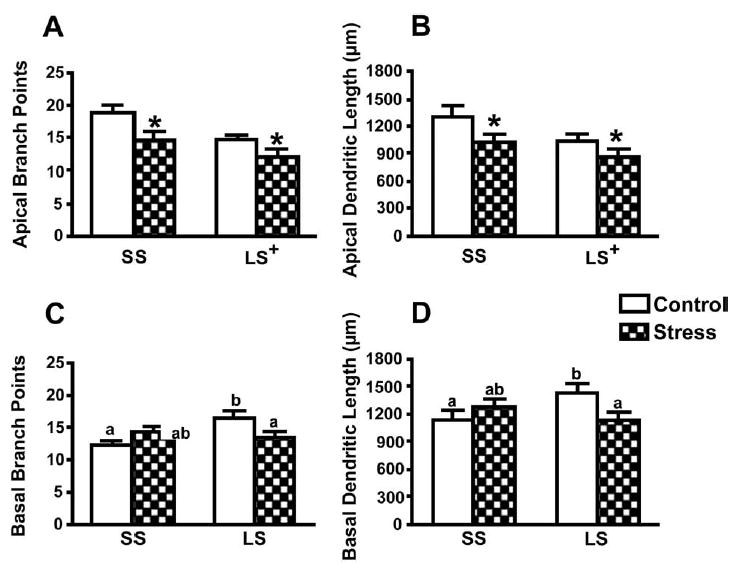
CA3 apical and basal dendritic morphology. Chronic restraint decreased both the apical branch points (A) and branch length (B). In addition, SS cells had more apical branch points and overall apical branch length compared with LS cells for both stressed and control conditions. Chronic restraint reduced both LS basal branch points (C) and length (D) compared with SS branch points and length. Data points represent group means ± S.E.M. * P<0.05 stress vs control. + P<0.05 SS vs. LS. For basal data, statistical significance is indicated by means with different letters (n=12 rats/group).
CA3 basal dendritic arborization
Chronic stress reduced branch points and length of LS, but not SS, basal arbors (Fig. 2C, D). A 2 × 2 mixed-factor ANOVA (treatment × cell type) indicated a significant interaction between treatment and cell type for branch points, F(1,22) = 9.59, P<0.01, and branch length, F(1,22) = 5.96, P<0.05. Further analyses using separate one-way ANO-VAs for the control and stress conditions demonstrated that indeed, chronic restraint specifically decreased LS basal dendritic properties without affecting SS basal dendrites. All other effects were not significant (P>0.10). Additionally, Pearson correlations failed to demonstrate any significant relationships between CA3 basal dendritic properties and differences scores, P>0.10.
CA1 apical and basal dendritic arborization
CA1 apical and basal dendritic arborization were unaffected by the treatment condition. There were no significant main effects or interactions for CA1 branch points or branch lengths (P>0.10; Table 1).
Table 1.
CA1 apical and basal dendritic morphology (means ± S.E.M.)
| Apical
|
Basal
|
|||
|---|---|---|---|---|
| Branch points | Branch length (μm) | Branch points | Branch length (μm) | |
| Control | 24.8 ± 1.4 | 1624.7 ± 120.7 | 16.9 ± 0.9 | 1445.7 ± 86.2 |
| Stress | 21.9 ± 1.7 | 1475.0 ± 108.7 | 16.2 ± 0.8 | 1460.3 ± 38.73 |
CA1 dendritic spines
A one-way ANOVA for treatment showed no significant effects for apical (groups means: control = 9.7 ± 7, stress = 10.8 ± 0.7) or basal CA1 spine densities (control = 9.8 ± 0.7, stress = 9.8 ± 0.8, P’s>0.10). However, chronic stress significantly influenced basal spine shape (Fig. 3). Chronically restrained rats exhibited a significantly greater proportion of basal spines with heads compared with controls, F(1,18) = 8.56, P<0.01. Chronic stress did not alter spine shape on apical dendrites (0.80 ± 0.02, 0.84 ± 0.04, for proportion of spines with heads in control and stress groups, respectively). No other effects were significant, P>0.10.
Fig. 3.
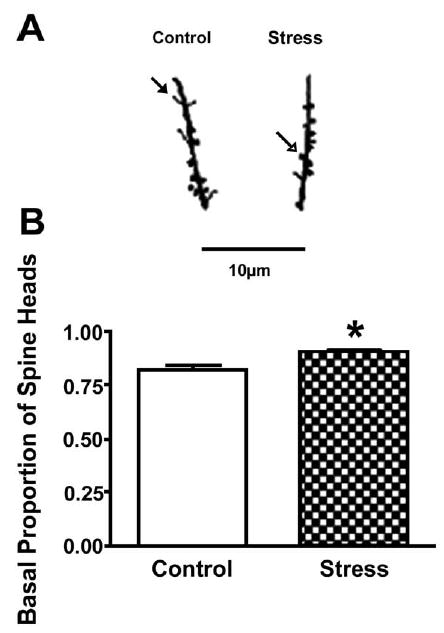
Basal CA1 dendritic spine proportions (heads to total). (A) Camera lucida drawings (1250×) represent CA1 basal dendritic spines from control and stress groups. The closed arrow points to an example of a headless spine whereas the open arrow points to a spine head. (B) Stressed rats had significantly more basal dendritic spines with heads compared with controls. Data points represent group means ± S.E.M. * P<0.05 (n = 10 rats/group).
Effects of chronic stress on the Y-maze
A one-way ANOVA for treatment showed no statistical difference for total entries between groups for the full five minutes, P>0.05. However, a recent study demonstrated an interaction between sex and minute for entries, with the greatest locomotor activity for female rats occurring within the first minute (Conrad et al., 2003). Due to this precedent and a possible indication of female habituation to the Y-maze, a two-way mixed-factor ANOVA (treatment × minutes) was conducted to analyze behavior in the first minute compared with the subsequent four minutes. All groups made significantly more entries during the first minute compared with the subsequent minutes, F(1,75) = 104.80, P<0.001. In addition, restrained rats made significantly fewer entries (3.5 ± 0.2) than their control counterparts (4.2 ± 0.2) during the first minute, F(1,75) = 5.14, P<0.05.
All groups entered the novel arm significantly more than the other arm during minute 1, according to a Wilcoxon matched pairs test (P<.01; Fig. 4A). Moreover, a one-way ANOVA for treatment showed a significant effect, F(1,75) = 4.32, P<0.05, with chronically restrained rats exhibiting higher positive difference scores compared with controls (Fig. 4B).
Fig. 4.
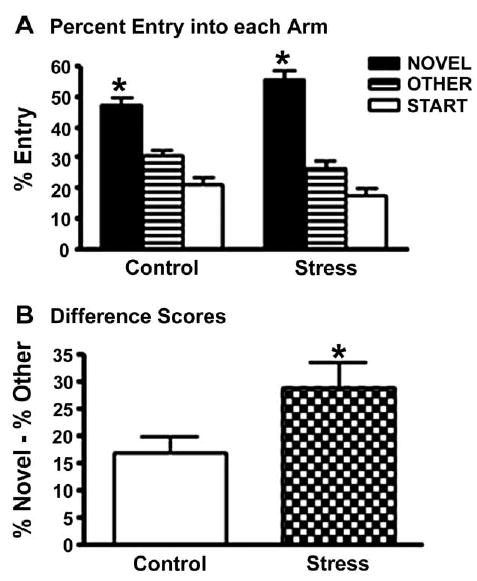
Influence of chronic stress on Y-maze performance. (A) Both groups entered the novel arm more than the other arm, * P<0.05 Novel vs. Other. (B) Stressed rats had significantly greater positive difference scores compared with control rats, * P<0.05, stress vs. control. Data points represent group means ± S.E.M. (control n = 39, stress n = 38).
Spearman correlations performed between CA3 dendritic retraction and behavior showed no statistically significant results for morphological correlations with total entries or difference scores, P>0.10. However, a correlation between CA1 basal spine head proportion (spines with heads/total spines) and difference score on the Y-maze did demonstrate a significant, positive relationship, rs(8) = 0.54, P<0.05. As difference scores became more positive, the proportion of spine heads increased. The majority of rats with the greatest difference scores and spine heads represented rats from the restrained group (Fig. 5). There were no other significant effects, P>0.10.
Fig. 5.
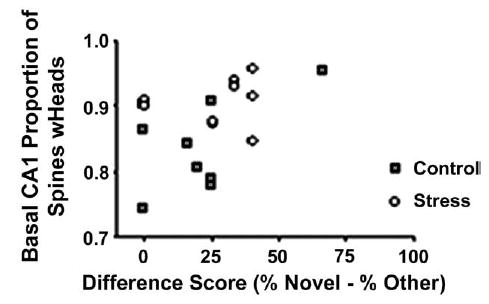
Correlation between Y-maze difference scores and proportion of CA1 basal spine heads. There was a significant, positive relationship for greater difference scores on the Y-maze (i.e. preference for the novel arm) to be associated with higher proportions of CA1 dendritic spines with heads, rs(8) = .54, P<0.05 (n = 10 rats/group).
Body weights
As predicted, restrained rats gained weight more slowly than controls over 21 days of chronic restraint. A 2 × 4 mixed-factor ANOVA (treatment × day) revealed statistically significant main effects of treatment, F(1,77) = 72.28, P<0.001 and day, F(3,231) = 212.6, P<0.001 and a significant interaction between treatment and day, F(3,231) = 49.44, P<0.001. Stressed females gained less weight over 21 days of restraint (268.9 ± 2.4 g, 291.7 ± 3.5 g, for days 1 and 21, respectively) compared with non-restrained controls (272.8 ± 2.8 g, 329.5 ± 3.1 g, for days 1 and 21, respectively).
DISCUSSION
This experiment investigated the effects of chronic stress on hippocampal morphology and spatial memory in OVX female rats. The results indicate that CA1 spine shape following chronic stress may parallel spatial memory in female rats despite robust CA3 dendritic retraction. Moreover, spatial memory can be intact or perhaps even enhanced in situations in which hippocampal CA3 dendritic retraction is present. Consequently, this study did not support the global interpretation that CA3 morphology predicts spatial memory, as demonstrated in male rats. Although numerous studies have suggested that ovarian hormones contribute to functional spatial memory in stressed females (Bowman et al., 2002; Bisagno et al., 2003), we have demonstrated that ovarian hormones are not necessary for intact spatial memory. Overall, these findings suggest that the CA1 region may play an important role in spatial memory ability and that it may override the contribution of the CA3 region following chronic stress in females.
Effects of chronic stress on hippocampal morphology
CA3
A previous study using intact females demonstrated CA3 dendritic retraction in the basal, but not apical region (Galea et al., 1997). In contrast, OVX females in the current study showed apical dendritic retraction in the CA3 region, which was consistent across both measures of branch points and total branch length. This apical dendritic retraction was similar to that seen in previous research using males (Watanabe et al., 1992c; Magarinos and McEwen, 1995a; Galea et al., 1997; Conrad et al., 1999; Sousa et al., 2000; Vyas et al., 2002). Perhaps OVX enabled CA3 apical dendritic retraction to occur by the removal of any potential neuroprotective effects of estrogen (Simpkins et al., 1997; Lee and McEwen, 2001; Bisagno et al., 2003). The current study also supported previous data that chronic stress reduced CA3 basal arbors in females (Galea et al., 1997). In addition, we present novel findings that stress-induced CA3 basal dendritic retraction is carried predominantly by LS and not SS neurons. This unique feature of stress targeting one cell type in females has also been observed in males, but with SS apical dendrites being more susceptible to stress than LS apical arbors (Lambert et al., 1998). These current findings indicate that having a bias for one cell type could potentially skew results, which may account for the discrepancy between studies. An alternate explanation for the discrepancies among studies (Galea et al., 1997) could be differences in Golgi methodology, but this interpretation is mitigated for several reasons. First, potential artifacts should be similar between experimental treatments. Second, our consistent measures of dendritic retraction derived from branch points and total branch length support our interpretation and confirm that SS cells are more complex than LS cells (Fitch et al., 1989). Finally, observing CA3 dendritic retraction using different Golgi procedures lends support that chronic stress alters hippocampal morphology.
CA1
Chronic stress did not affect spine density, but it increased the proportion of spine heads in the CA1 region. Spines change shape as they mature, most noticeably by developing a head at the end of the spine (Kasai et al., 2003). Large, more mature spines express high numbers of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepro-prionic acid receptors, greater glutamate sensitivity, and are associated with long-term potentiation (LTP), which is thought to reflect greater synaptic activity and memory function (Matsuzaki et al., 2001; Kasai et al., 2003). In addition, drugs that inhibit actin filaments within spine heads interfere with LTP, which is thought to be important for memory coding and consolidation within the hippocampus (Mesches et al., 1999; Ackermann and Matus, 2003). While ovarian hormones and androgens induce changes in both spine density and shape in rodents (Gould et al., 1990; Woolley et al., 1997; Leranth et al., 2004; Li et al., 2004; Gonzalez-Burgos et al., 2005), these data show that chronic stress modulates CA1 spine shape as well.
Effects of chronic stress on the Y-maze
Chronically-stressed rats showed greater novel arm preference compared with controls on the spatial recognition memory component of the Y-maze. Stress may have enhanced performance by interfering with habituation to the novel arm. This interpretation is supported by impaired habituation to novel environments in male rats following psychosocial stress (Park et al., 2001). Another interpretation is that chronic stress facilitated females’ spatial memory, similar to the facilitative effects found in the radial arm maze and object placement tasks (Bowman et al., 2001, 2002, 2003; Beck and Luine, 2002). This diversity of tasks that use novelty or appetitive training suggests that performance in females is facilitated by chronic stress through neural mechanisms underlying spatial memory.
Non-mnemonic mechanisms, such as changes in motor ability or motivation, may have also contributed to the facilitated performance of stressed females. During the five minutes of testing, control and stressed rats made a similar number of entries in the Y-maze, suggesting that both groups had similar motor behavior and motivational ability. Within minute 1, however, stressed females made fewer entries than controls. These data indicate that fewer entries are not necessarily synonymous with reduced motor ability or motivation because rats showing the best novel arm preference made the fewest entries.
Another interpretation is that stress influenced the preference for novelty and altered performance in the Y-maze by affecting entries into the novel arm. However, male rats that are impaired in the traditional version of the Y-maze successfully seek out the novel arm when salient, intrinsic cues are provided or when the intertrial-interval is reduced to 1 min (Wright and Conrad, 2005; Kleen et al., 2004). Whether chronic stress enhances novelty-seeking behavior in females has yet to be determined.
Differences in the perception of restraint between males and females may explain the lack of impairment in female spatial memory ability. Some reports have shown that males have less weight gain following chronic stress than females (Watanabe et al., 1992c; Magarinos and McEwen, 1995b; Conrad et al., 2003, 2004b), which suggests that males may perceive restraint as more stressful than females. However, the females in the current study displayed robust CA3 hippocampal dendritic retraction in both the apical and basal regions of the hippocampus, which is more extensive than the amount of stress-induced CA3 dendritic retraction previously reported for males using the same restraint protocol (Watanabe et al., 1992c; Magarinos and McEwen, 1995a; Conrad et al., 1999). Therefore, the females in our study appear to have perceived restraint as stressful as males.
Neurochemical and hormonal changes following OVX and/or chronic stress may have contributed to spatial memory performance. Young and aged rats who experience long-term OVX (1.5–6 months) display significantly better performance in the water radial-arm maze than their sham counterparts, most likely from the hormonal shift occurring after OVX (Bimonte-Nelson et al., 2003). Chronic restraint stress affects the dopaminergic system in the prefrontal cortex, and these stress-dependent changes are not demonstrated in gonadally-intact stressed females (Bowman et al., 2002). Three weeks following OVX, females have a faster recovery of adrenocorticotropin hormone (ACTH) and CORT response to shock stress compared with females given chronic estrogen replacement (Burgess and Handa, 1992). In general, females have higher basal levels of CORT and release more CORT than males in response to stress (Atkinson and Waddell, 1997; Galea et al., 1997; Rivier, 1999; Kitraki et al., 2004). At the same time, female rats habituate more quickly to restraint stress compared with males (Bowman et al., 2001; Luine, 2002), but these sex differences in CORT production are not always observed (Figueiredo et al., 2002; Conrad et al., 2004a). In addition, chronic stress sex-dependently alters the two CORT receptors within the CA1 region of the hippocampus (Kitraki et al., 2004). Any one or combination of these changes may influence the success of chronically stressed females in spatial memory tasks. Moreover, these fluctuations may affect other areas in the brain, in addition to hippocampal CA3 and CA1 cells.
The lack of an estrogen influence on hippocampal morphology and behavior
The similarity of CA1 spine densities across experimental groups may seem surprising, as it has been well documented that acute estrogen treatment increases apical CA1 spine density (Woolley and McEwen, 1993; Woolley et al., 1997). Within 48 h of administering 17β-estradiol, CA1 spine density increases and these maximal levels are maintained for an additional 48 h after estrogen is metabolized, then density slowly declines over the next few days (Woolley and McEwen, 1993; Woolley, 1998). While the 17β-estradiol dose used in this study (0.1 mL of 5 μg) is similar to that which has been used in other behavioral paradigms (Chesler and Juraska, 2000; Korol and Kolo, 2002), this dose is too low to fully reproduce increases in CA1 apical spine density which have been demonstrated in morphological studies (0.1 mL of 10 μg; Gould et al., 1990; Woolley and McEwen, 1993; Woolley, 1998). Therefore, estrogen treatments used in future research will need to include higher doses in order to determine a more clear relationship between changes in the brain and subsequent behavior. In addition to dose, differences between past research and the present study may involve a difference in behavioral training and handling procedures. For example, significant differences in CA1 spine densities between estrogen and vehicle treated rats disappear following handling for 5 min/day/14 days (Garza-Meilandt et al., 2002). Thus handling, combined with estrogen replacement, may have retarded OVX-induced changes in spine density in our rats.
Estrogen’s inability to alter Y-maze performance should also be further explored. Acute estrogen replacement can be given 3 weeks to 4 months after OVX to observe changes in memory (Korol and Kolo, 2002; Sandstrom and Williams, 2004). Administering 17β-estradiol to OVX females either 72 and 48 h, or 48 and 24 h before behavioral assessment, which is consistent with our time frame, can either improve or impair spatial memory, depending on the task, compared with controls (Frye, 1995; Sandstrom and Williams, 2001; Korol and Kolo, 2002). Perhaps our handling procedure may have negated estrogen’s effects on the Y-maze because of the enriched setting. Environmental enrichment can increase spatial memory ability on several tasks (Fernandez-Teruel et al., 1997; Gresack and Frick, 2004). However, a recent finding demonstrated that while mice treated with estrogen had improved spatial memory, estrogen combined with previous exposure to an enriched environment did not alter object recognition and actually impaired work memory acquisition (Gresack and Frick, 2004). Although this study used mice and an enriched environment protocol lasting several months, the results provide an interesting possibility. Since all of the rats in this study were exposed to the same handling and environmental cues and all animals had intact spatial memory, we cannot separate possible interactions between estrogen and the environment.
Significance
Our research contributes to growing evidence demonstrating that CA3 hippocampal morphology does not always predict spatial memory function. Although CA3 dendritic retraction is associated with memory deficits in males, chronically-stressed male rats exhibit increased hippocampal-dependent contextual fear conditioning compared with controls, despite CA3 dendritic retraction (Conrad et al., 1999, 2001). In addition, inhibiting CORT synthesis with metyrapone on the day of training prevents stress-induced spatial memory deficits when dendritic retraction should be present (Wright et al., 2004). Finally, three weeks of CORT treatment causes CA3 dendritic retraction, but spatial memory remains intact on the Y-Maze (Coburn-Litvak et al., 2003). Therefore, CA3 dendritic retraction does not always parallel spatial memory deficits in males, and our findings with females contribute to this growing literature. Our study indicates that stress-induced changes in CA1 spine shape are associated with enhanced spatial memory in OVX female rats. These data are consistent with studies showing that changes in CA1 spines, following estrogen treatment, parallel spatial memory retention (Sandstrom and Williams, 2004). Together these findings suggest that the presence of mature spine heads on CA1 hippocampal dendrites may play a more critical role than CA3 morphology in predicting spatial memory in females.
Acknowledgments
This work was funded by MH64727 (Conrad). The contributions of following individuals are gratefully acknowledged: Rudy Bellani, Meghan Ellis, Juan Gomez, Gillian Hamilton, James Harman, Jonathan Kleen, Jamie Jackson, Scott Seganti, Sergey Tsekhanov, and Lindsay Wieczorek.
References
- Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6:1194–1200. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: Sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- Bannister NJ, Larkman AU. Dendritic morphology of CA1 pyramidal neurones from the rat hippocampus: I. Branching patterns. J Comp Neurol. 1995;360:150–160. doi: 10.1002/cne.903600111. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav. 1987;27:53–59. doi: 10.1016/0091-3057(87)90476-x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117:1395–1406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Bowman R, Luine V. Functional aspects of estrogen neuroprotection. Endocrine. 2003;21:33–41. doi: 10.1385/endo:21:1:33. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Horm Behav. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learn Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS (2003) Applied multiple regression/correlation analysis for the behavioral sciences. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav. 2004a;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wise LS. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004b;125:759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Mauldin-Jourdain ML, Hobbs RJ. Metyrapone reveals that previous chronic stress differentially impairs hippocampal-dependent memory. Stress. 2001;4:305–318. doi: 10.3109/10253890109014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Escorihuela RM, Castellano B, Gonzalez B, Tobena A. Neonatal handling and environmental enrichment effects on emotionality, novelty/reward seeking, and age-related cognitive and hippocampal impairments: focus on the Roman rat lines. Behav Genet. 1997;27:513–526. doi: 10.1023/a:1021400830503. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- Fitch JM, Juraska JM, Washington LW. The dendritic morphology of pyramidal neurons in the rat hippocampal CA3 area. I. Cell types. Brain Res. 1989;479:105–114. doi: 10.1016/0006-8993(89)91340-1. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garza-Meilandt A, Cantu RE, Stone KK, Zamora DA, Jaffe DB, Claiborne BJ (2002) Effects of estrogen treatment and handling on spatial learning and spine densities on hippocampal CA1 neurons. In: 2002 Abstract Viewer: Society for Neuroscience. No. 574.14, Washington, DC.
- Gonzalez-Burgos I, Alejandre-Gomez M, Cervantes M. Spine-type densities of hippocampal CA1 neurons vary in proestrus and estrus rats. Neurosci Lett. 2005;379:52–54. doi: 10.1016/j.neulet.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W. Gender differences in depression: perspectives from neuropsychology. J Affect Disord. 1993;29:129–143. doi: 10.1016/0165-0327(93)90028-i. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Sitomer MT, Killeen PR, Conrad CD (2004) Changes in operant behavior during and after chronic stress: A look at motivation, motor ability, and memory. In: 2004 Abstract Viewer: Society for Neuroscience. No. 426.6, San Diego.
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Buckelew SK, Staffiso-Sandoz G, Gaffga S, Carpenter W, Fisher J, Kinsley CH. Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiol Behav. 1998;65:43–49. doi: 10.1016/s0031-9384(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MJ, Wang PS. Relative and combined effects of estradiol and prolactin on corticosterone secretion in ovariectomized rats. Chin J Physiol. 2003;46:103–109. [PubMed] [Google Scholar]
- Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994a;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Stress-dependent impairments of spatial memory. Role of 5-HT. Ann N Y Acad Sci. 1994b;746:403–404. doi: 10.1111/j.1749-6632.1994.tb39268.x. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995a;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995b;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Res. 1998;809:314 –318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesches MH, Fleshner M, Heman KL, Rose GM, Diamond DM. Exposing rats to a predator blocks primed burst potentiation in the hippocampus in vitro. J Neurosci. 1999;19:RC18. doi: 10.1523/JNEUROSCI.19-14-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa M, Bundman MC, Greenberger V, Segal M. Morphological analysis of dendritic spine development in primary cultures of hippocampal neurons. J Neurosci. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biol Psychiatry. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol Biochem Behav. 1999;64:739–751. doi: 10.1016/s0091-3057(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesteone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Hippocampal atrophy in major depression: a result of depression-induced neurotoxicity? Mol Psychiatry. 1996;1:298–299. [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer’s disease. Am J Med. 1997;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992b;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992c;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Ter Horst GJ, Roos MH, Kuipers SD, Trentani A, den Boer JA. Gender-specific effects of social housing in rats after chronic mild stress exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:21–30. doi: 10.1016/s0278-5846(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140 –148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Lightner EN, Bellani R, Harman JS, Conrad CD (2004) Chronic stress-induced spatial memory deficits are prevented by metyrapone treatment on the day of behavioral assessment. In: 2004 Abstract Viewer: Society for Neuroscience. No. 316.3, San Diego.


