Abstract
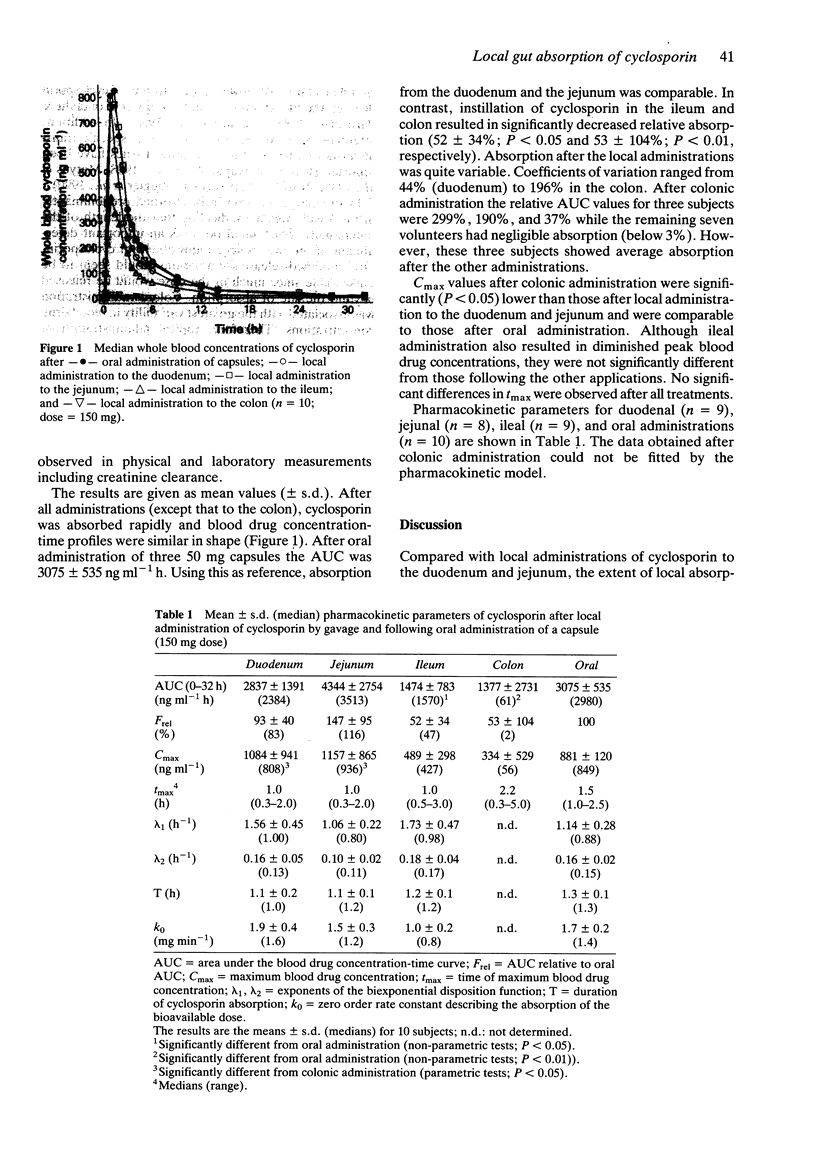
1. An emulsion preparation of cyclosporin was administered locally to different parts of the small and large intestine by gavage: to the duodenum (opposite to the papilla of Vater), jejunum (150 cm distal to the teeth), ileum (300 cm distal to the teeth), and to the colon descendens (30 cm proximal to the anus). 2. The bioavailability of cyclosporin after these instillations was compared with that after oral administration of a hard gelatine capsule formulation. 3. Cyclosporin was found to be absorbed predominantly in the small intestine. This may have implications for dosage in patients with reduced absorptive surface area.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W., Fyock B., Gray S., Coln D., Hendrickse W., Siegel J., Belknap B., Hogge A., Benser M., Kennard B. Pediatric liver transplantation: the Dallas experience. Transplant Proc. 1987 Aug;19(4):3267–3276. [PubMed] [Google Scholar]
- Atkinson K., Biggs J. C., Britton K., Short R., Mrongovius R., Concannon A., Dodds A. Oral administration of cyclosporin A for recipients of allogeneic marrow transplants: implications of clinical gut dysfunction. Br J Haematol. 1984 Feb;56(2):223–231. doi: 10.1111/j.1365-2141.1984.tb03950.x. [DOI] [PubMed] [Google Scholar]
- Ball P. E., Munzer H., Keller H. P., Abisch E., Rosenthaler J. Specific 3H radioimmunoassay with a monoclonal antibody for monitoring cyclosporine in blood. Clin Chem. 1988 Feb;34(2):257–260. [PubMed] [Google Scholar]
- Brynskov J., Freund L., Thomsen O. O., Andersen C. B., Rasmussen S. N., Binder V. Treatment of refractory ulcerative colitis with cyclosporin enemas. Lancet. 1989 Apr 1;1(8640):721–722. doi: 10.1016/s0140-6736(89)92232-0. [DOI] [PubMed] [Google Scholar]
- Cefalu W. T., Pardridge W. M. Restrictive transport of a lipid-soluble peptide (cyclosporin) through the blood-brain barrier. J Neurochem. 1985 Dec;45(6):1954–1956. doi: 10.1111/j.1471-4159.1985.tb10557.x. [DOI] [PubMed] [Google Scholar]
- Frey F. J., Horber F. F., Frey B. M. Trough levels and concentration time curves of cyclosporine in patients undergoing renal transplantation. Clin Pharmacol Ther. 1988 Jan;43(1):55–62. doi: 10.1038/clpt.1988.11. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Benet L. Z. High-fat meals increase the clearance of cyclosporine. Pharm Res. 1990 Jan;7(1):46–48. doi: 10.1023/a:1015831408425. [DOI] [PubMed] [Google Scholar]
- Johnston A., Marsden J. T., Hla K. K., Henry J. A., Holt D. W. The effect of vehicle on the oral absorption of cyclosporin. Br J Clin Pharmacol. 1986 Mar;21(3):331–333. doi: 10.1111/j.1365-2125.1986.tb05200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine. N Engl J Med. 1989 Dec 21;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Keown P. A., Stiller C. R., Laupacis A. L., Howson W., Coles R., Stawecki M., Koegler J., Carruthers G., McKenzie N., Sinclair N. R. The effects and side effects of cyclosporine: relationship to drug pharmacokinetics. Transplant Proc. 1982 Dec;14(4):659–661. [PubMed] [Google Scholar]
- Kukongviriyapan V., Stacey N. H. Inhibition of taurocholate transport by cyclosporin A in cultured rat hepatocytes. J Pharmacol Exp Ther. 1988 Nov;247(2):685–689. [PubMed] [Google Scholar]
- Köhler E., Duberow-Drewe M., Drewe J., Ribes G., Loubatiéres-Mariani M. M., Mazer N., Gyr K., Beglinger C. Absorption of an aqueous solution of a new synthetic somatostatin analogue administered to man by gavage. Eur J Clin Pharmacol. 1987;33(2):167–171. doi: 10.1007/BF00544562. [DOI] [PubMed] [Google Scholar]
- Lemaire M., Pardridge W. M., Chaudhuri G. Influence of blood components on the tissue uptake indices of cyclosporin in rats. J Pharmacol Exp Ther. 1988 Feb;244(2):740–743. [PubMed] [Google Scholar]
- Lindholm A., Henricsson S., Lind M., Dahlqvist R. Intraindividual variability in the relative systemic availability of cyclosporin after oral dosing. Eur J Clin Pharmacol. 1988;34(5):461–464. doi: 10.1007/BF01046702. [DOI] [PubMed] [Google Scholar]
- Mehta M. U., Venkataramanan R., Burckart G. J., Ptachcinski R. J., Delamos B., Stachak S., Van Thiel D. H., Iwatsuki S., Starzl T. E. Effect of bile on cyclosporin absorption in liver transplant patients. Br J Clin Pharmacol. 1988 May;25(5):579–584. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátyus L., Balázs M., Aszalós A., Mulhern S., Damjanovich S. Cyclosporin A depolarizes cytoplasmic membrane potential and interacts with Ca2+ ionophores. Biochim Biophys Acta. 1986 May 29;886(3):353–360. doi: 10.1016/0167-4889(86)90170-9. [DOI] [PubMed] [Google Scholar]
- Ptachcinski R. J., Venkataramanan R., Burckart G. J. Clinical pharmacokinetics of cyclosporin. Clin Pharmacokinet. 1986 Mar-Apr;11(2):107–132. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- Ptachcinski R. J., Venkataramanan R., Rosenthal J. T., Burckart G. J., Taylor R. J., Hakala T. R. The effect of food on cyclosporine absorption. Transplantation. 1985 Aug;40(2):174–176. doi: 10.1097/00007890-198508000-00013. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Terres G. Cyclosporine A inhibits Ca2+-dependent stimulation of the Na+/H+ antiport in human T cells. J Cell Biol. 1986 Aug;103(2):457–463. doi: 10.1083/jcb.103.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchuk R. J., Awni W. M. Absorption of cyclosporine from rabbit small intestine in situ. J Pharm Sci. 1986 Dec;75(12):1151–1156. doi: 10.1002/jps.2600751207. [DOI] [PubMed] [Google Scholar]
- Venkataramanan R., Burckhart G. J., Ptachcinski R. J. Pharmacokinetics and monitoring of cyclosporine following orthotopic liver transplantation. Semin Liver Dis. 1985 Nov;5(4):357–368. doi: 10.1055/s-2008-1040633. [DOI] [PubMed] [Google Scholar]
- Whitington P. F., Emond J. C., Whitington S. H., Broelsch C. E., Baker A. L. Small-bowel length and the dose of cyclosporine in children after liver transplantation. N Engl J Med. 1990 Mar 15;322(11):733–738. doi: 10.1056/NEJM199003153221105. [DOI] [PubMed] [Google Scholar]
- Williams J. D., Salaman J. R., Griffin P. J., Hillis A. N., Ross W., Williams G. T. Malabsorption of cyclosporin in renal transplant recipient with Crohn's disease. Lancet. 1987 Apr 18;1(8538):914–915. doi: 10.1016/s0140-6736(87)92879-0. [DOI] [PubMed] [Google Scholar]
- Ziegler K., Frimmer M., Fritzsch G., Koepsell H. Cyclosporin binding to a protein component of the renal Na(+)-D-glucose cotransporter. J Biol Chem. 1990 Feb 25;265(6):3270–3277. [PubMed] [Google Scholar]


