Abstract
1 In total 1999 consecutive admissions to six medical wards were subjected to a prospective high-intensity drug event monitoring scheme to assess the extent and pattern of admissions caused by adverse drug reactions (ADRs) or dose related therapeutic failures (TF), in a population-based design. The wards were sub-specialised in general medicine, geriatrics, endocrinology, cardiology, respiratory medicine and gastroenterology.
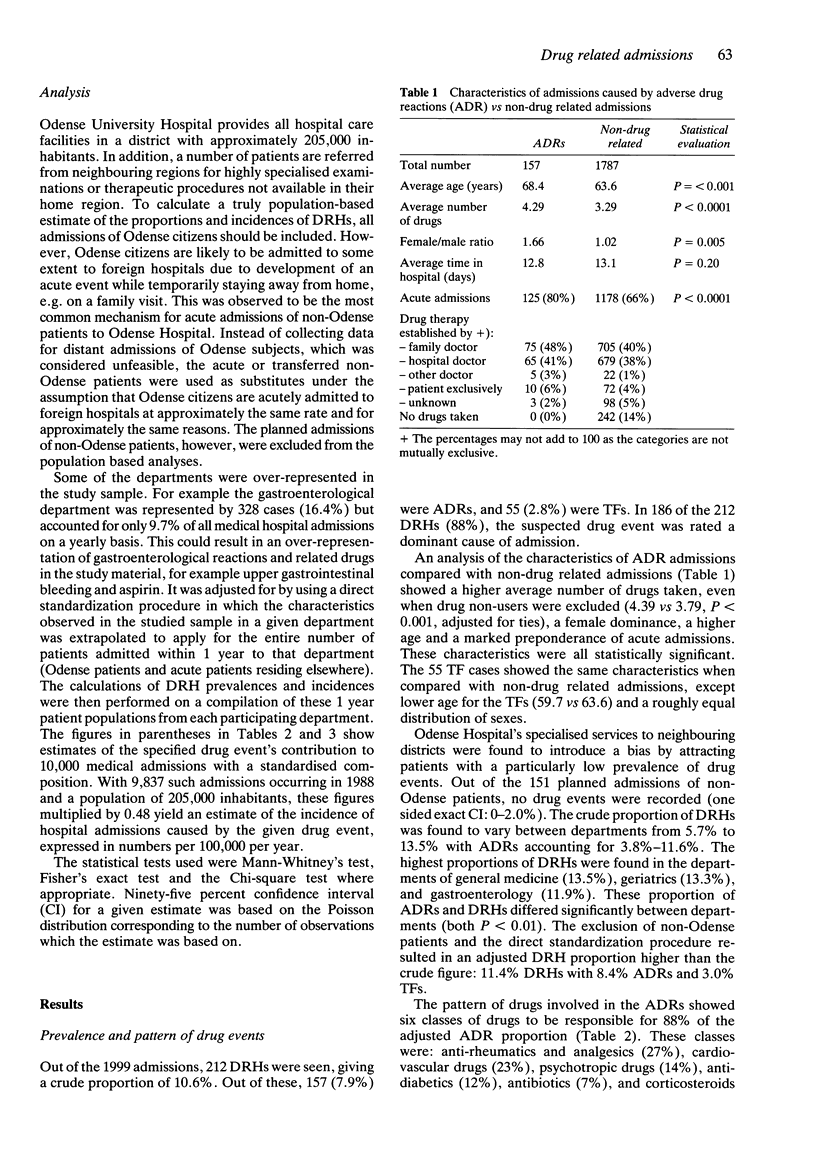
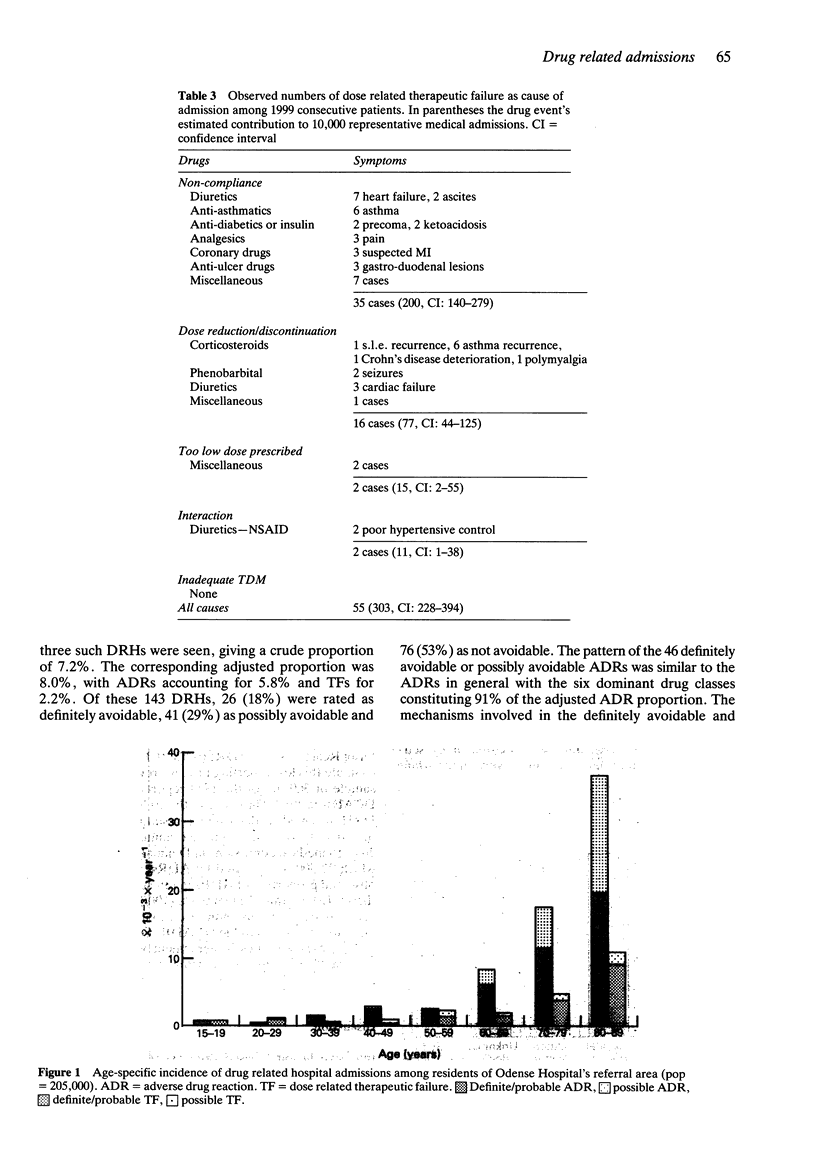
2 Considering definite, probable and possible drug events, the prevalence of drug related hospital admissions was 11.4% of which 8.4% were caused by ADRs and 3.0% by TFs. There were large inter-department differences.
3 The six classes of drugs most frequently involved in admissions caused by ADRs were anti-rheumatics and analgesics (27%), cardiovascular drugs (23%), psychotropic drugs (14%), anti-diabetics (12%), antibiotics (7%), and corticosteroids (5%). Non-compliance accounted for 66% of the TFs with diuretics and anti-asthmatics most frequently involved.
4 The pattern of drugs involved in ADRs was compared with the regional drug sales statistics. Drugs with a particularly high rate of ADR related admissions per unit dispensed were nitrofurantoin and insulin (617 and 182 admissions per 1,000,000 defined daily doses), while low rates were seen for diuretics and benzodiazepines (10 and 7 admissions per 1,000,000 defined daily doses). Confidence intervals were wide.
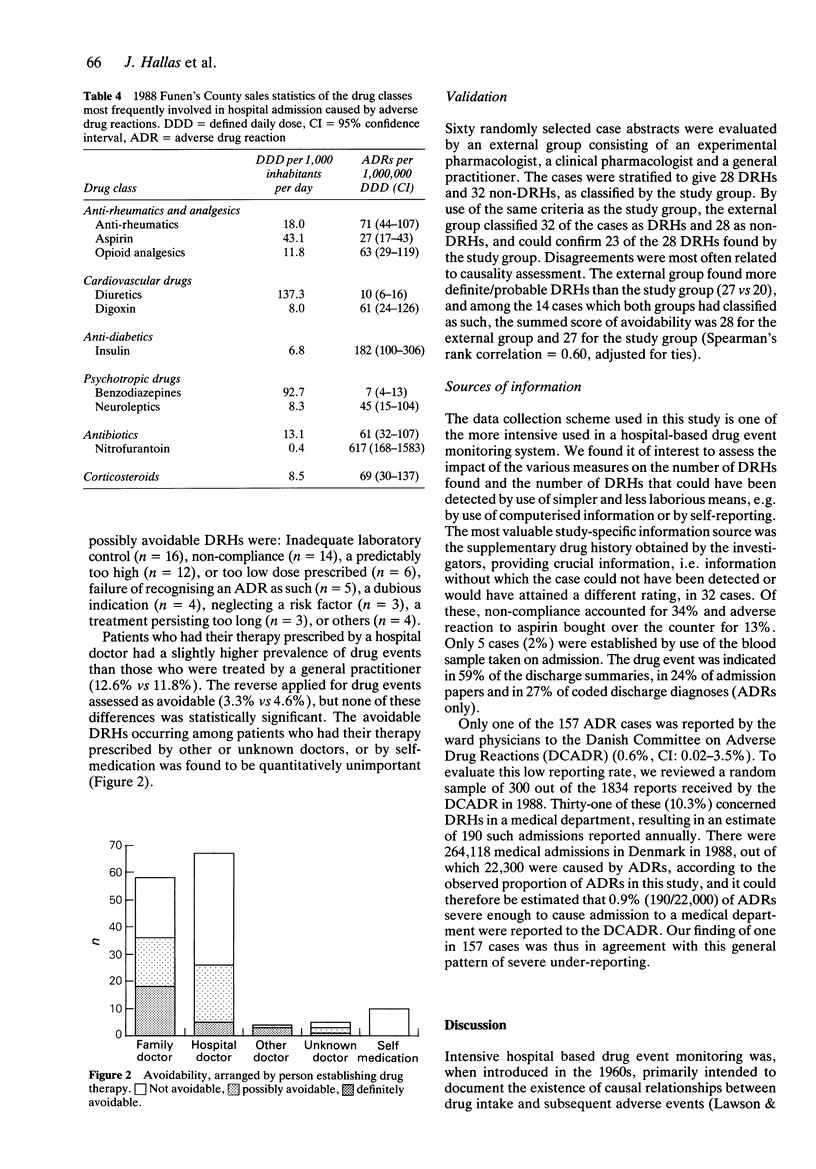
5 Patients who had their therapy prescribed by a hospital doctor had a slightly higher prevalence of drug events than those who were treated by a general practitioner (12.6% vs 11.8%). The reverse applied for drug events assessed as avoidable (3.3% vs 4.6%). Although these differences were not statistically significant, it may suggest general practitioners as the appropriate target for interventive measures.
6 Only one ADR was reported to The Danish Committee on Adverse Drug Reactions, indicating a severe under-reporting and a potential for gross selectivity. The data collection system used here is expensive, but may be modified to provide reliably representative data on serious ADRs in a more cost-effective fashion.
Keywords: adverse drug reactions, hospital admission, non-compliance, spontaneous reporting
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arneborn P., Palmblad J. Drug-induced neutropenia--a survey for Stockholm 1973-1978. Acta Med Scand. 1982;212(5):289–292. doi: 10.1111/j.0954-6820.1982.tb03216.x. [DOI] [PubMed] [Google Scholar]
- Beard K., Perera D. R., Jick H. Drug-induced parenchymal renal disease in outpatients. J Clin Pharmacol. 1988 May;28(5):431–435. doi: 10.1002/j.1552-4604.1988.tb05755.x. [DOI] [PubMed] [Google Scholar]
- Bergman U., Elmes P., Halse M., Halvorsen T., Hood H., Lunde P. K., Sjöqvist F., Wade O. L., Westerholm B. The measurement of drug consumption. Drugs for diabetes in Northern Ireland, Norway and Sweden. Eur J Clin Pharmacol. 1975 Feb 28;8(2):83–89. doi: 10.1007/BF00561555. [DOI] [PubMed] [Google Scholar]
- Bergman U., Wiholm B. E. Drug-related problems causing admission to a medical clinic. Eur J Clin Pharmacol. 1981;20(3):193–200. doi: 10.1007/BF00544597. [DOI] [PubMed] [Google Scholar]
- Böttiger L. E., Strandberg I., Westerholm B. Drug-induced febrile mucocutaneous syndrome with a survey of the literature. Acta Med Scand. 1975 Sep;198(3):229–233. [PubMed] [Google Scholar]
- Caranasos G. J., Stewart R. B., Cluff L. E. Drug-induced illness leading to hospitalization. JAMA. 1974 May 6;228(6):713–717. [PubMed] [Google Scholar]
- Cooke D. I., van der Merwe W., Pudifin D. J. Hospital admissions for adverse reactions to drugs and deliberate self-poisoning. S Afr Med J. 1985 May 11;67(19):770–772. [PubMed] [Google Scholar]
- Ghose K. Hospital bed occupancy due to drug-related problems. J R Soc Med. 1980 Dec;73(12):853–856. doi: 10.1177/014107688007301207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. P. Survey of the spontaneous adverse drug reaction reporting schemes in fifteen countries. Br J Clin Pharmacol. 1986;22 (Suppl 1):83S–100S. doi: 10.1111/j.1365-2125.1986.tb02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallas J., Haghfelt T., Gram L. F., Grodum E., Damsbo N. Drug related admissions to a cardiology department; frequency and avoidability. J Intern Med. 1990 Oct;228(4):379–384. doi: 10.1111/j.1365-2796.1990.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Hallas J., Harvald B., Gram L. F., Grodum E., Brøsen K., Haghfelt T., Damsbo N. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med. 1990 Aug;228(2):83–90. doi: 10.1111/j.1365-2796.1990.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Hallas J., Jensen K. B., Grodum E., Damsbo N., Gram L. F. Drug-related admissions to a department of medical gastroenterology. The role of self-medicated and prescribed drugs. Scand J Gastroenterol. 1991 Feb;26(2):174–180. doi: 10.3109/00365529109025028. [DOI] [PubMed] [Google Scholar]
- Hurwitz N. Admissions to hospital due to drugs. Br Med J. 1969 Mar 1;1(5643):539–540. doi: 10.1136/bmj.1.5643.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman W. H. Prescription-event monitoring. Br Med J (Clin Res Ed) 1982 Sep 18;285(6344):809–810. doi: 10.1136/bmj.285.6344.809-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna L. The Halcion story: trial by media. Lancet. 1980 Apr 12;1(8172):815–816. doi: 10.1016/s0140-6736(80)91306-9. [DOI] [PubMed] [Google Scholar]
- Levy M., Kewitz H., Altwein W., Hillebrand J., Eliakim M. Hospital admissions due to adverse drug reactions: a comparative study from Jerusalem and Berlin. Eur J Clin Pharmacol. 1980 Jan;17(1):25–31. doi: 10.1007/BF00561673. [DOI] [PubMed] [Google Scholar]
- Miller R. R. Hospital admissions due to adverse drug reactions. A report from the Boston Collaborative Drug Surveillance Program. Arch Intern Med. 1974 Aug;134(2):219–223. [PubMed] [Google Scholar]
- Petri H., de Vet H. C., Naus J., Urquhart J. Prescription sequence analysis: a new and fast method for assessing certain adverse reactions of prescription drugs in large populations. Stat Med. 1988 Nov;7(11):1171–1175. doi: 10.1002/sim.4780071110. [DOI] [PubMed] [Google Scholar]
- Rawlins M. D. Spontaneous reporting of adverse drug reactions. II: Uses. Br J Clin Pharmacol. 1988 Jul;26(1):7–11. doi: 10.1111/j.1365-2125.1988.tb03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom B. L., Morse M. L. Use of computerized databases to survey drug utilization in relation to diagnoses. Acta Med Scand Suppl. 1988;721:13–20. doi: 10.1111/j.0954-6820.1987.tb05372.x. [DOI] [PubMed] [Google Scholar]


