Abstract
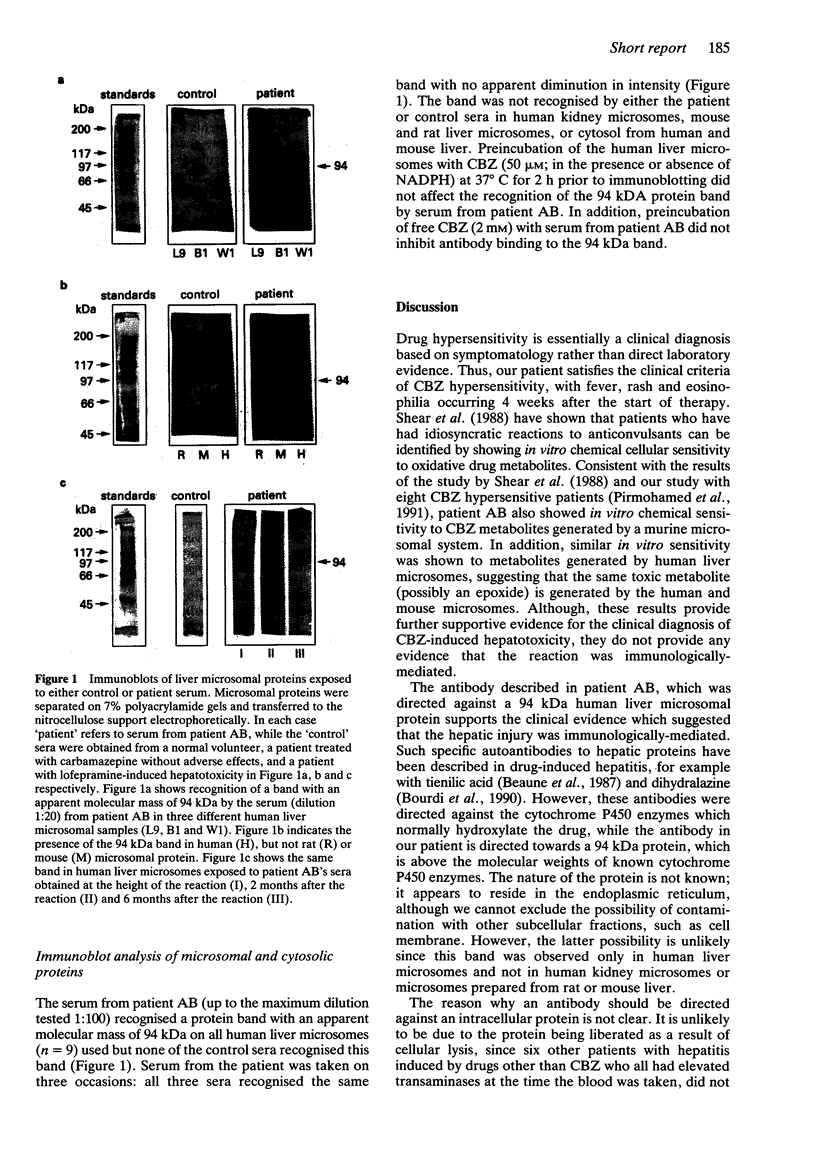
A 16-year old patient with carbamazepine-induced hepatotoxicity, associated with the hypersensitivity manifestations of fever, rash and eosinophilia, is described. Mononuclear leucocytes from the patient were more sensitive to oxidative metabolites of carbamazepine generated by induced murine and human hepatic microsomes, than cells from controls. On immunoblot analysis, serum from the patient recognised a single protein band (94 kDa) on human liver microsomes, but none out of 25 control sera recognised this band. No bands were recognised by the patient serum on human kidney microsomes or on microsomes from mouse and rat liver.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaune P., Dansette P. M., Mansuy D., Kiffel L., Finck M., Amar C., Leroux J. P., Homberg J. C. Human anti-endoplasmic reticulum autoantibodies appearing in a drug-induced hepatitis are directed against a human liver cytochrome P-450 that hydroxylates the drug. Proc Natl Acad Sci U S A. 1987 Jan;84(2):551–555. doi: 10.1073/pnas.84.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M., Larrey D., Nataf J., Bernuau J., Pessayre D., Iwasaki M., Guengerich F. P., Beaune P. H. Anti-liver endoplasmic reticulum autoantibodies are directed against human cytochrome P-450IA2. A specific marker of dihydralazine-induced hepatitis. J Clin Invest. 1990 Jun;85(6):1967–1973. doi: 10.1172/JCI114660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss F. E., Langer D. H. Hepatic considerations in the use of antiepileptic drugs. Epilepsia. 1987;28 (Suppl 2):S23–S29. doi: 10.1111/j.1528-1157.1987.tb05768.x. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Aw T. Y., Simon F. R., Stolz A. Drug-induced hepatotoxicity. Ann Intern Med. 1986 Jun;104(6):826–839. doi: 10.7326/0003-4819-104-6-826. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Park B. K., Coleman J. W., Kitteringham N. R. Drug disposition and drug hypersensitivity. Biochem Pharmacol. 1987 Mar 1;36(5):581–590. [PubMed] [Google Scholar]
- Park B. K. Metabolic basis of adverse drug reactions. J R Coll Physicians Lond. 1986 Jul;20(3):195–200. [PMC free article] [PubMed] [Google Scholar]
- Pellock J. M. Carbamazepine side effects in children and adults. Epilepsia. 1987;28 (Suppl 3):S64–S70. doi: 10.1111/j.1528-1157.1987.tb05780.x. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M., Graham A., Roberts P., Smith D., Chadwick D., Breckenridge A. M., Park B. K. Carbamazepine-hypersensitivity: assessment of clinical and in vitro chemical cross-reactivity with phenytoin and oxcarbazepine. Br J Clin Pharmacol. 1991 Dec;32(6):741–749. [PMC free article] [PubMed] [Google Scholar]
- Purba H. S., Maggs J. L., Orme M. L., Back D. J., Park B. K. The metabolism of 17 alpha-ethinyloestradiol by human liver microsomes: formation of catechol and chemically reactive metabolites. Br J Clin Pharmacol. 1987 Apr;23(4):447–453. doi: 10.1111/j.1365-2125.1987.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Maggs J. L., Lambert C., Kitteringham N. R., Park B. K. An in vitro study of the microsomal metabolism and cellular toxicity of phenytoin, sorbinil and mianserin. Br J Clin Pharmacol. 1988 Nov;26(5):577–588. doi: 10.1111/j.1365-2125.1988.tb05298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear N. H., Spielberg S. P. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J Clin Invest. 1988 Dec;82(6):1826–1832. doi: 10.1172/JCI113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska J. M., Ivanyi L. In vitro lymphocyte proliferation by carbamazepine, carbamazepine-10, 11-epoxide, and oxcarbazepine in the diagnosis of drug-induced hypersensitivity. J Allergy Clin Immunol. 1988 Jul;82(1):110–115. doi: 10.1016/0091-6749(88)90059-0. [DOI] [PubMed] [Google Scholar]



