Abstract
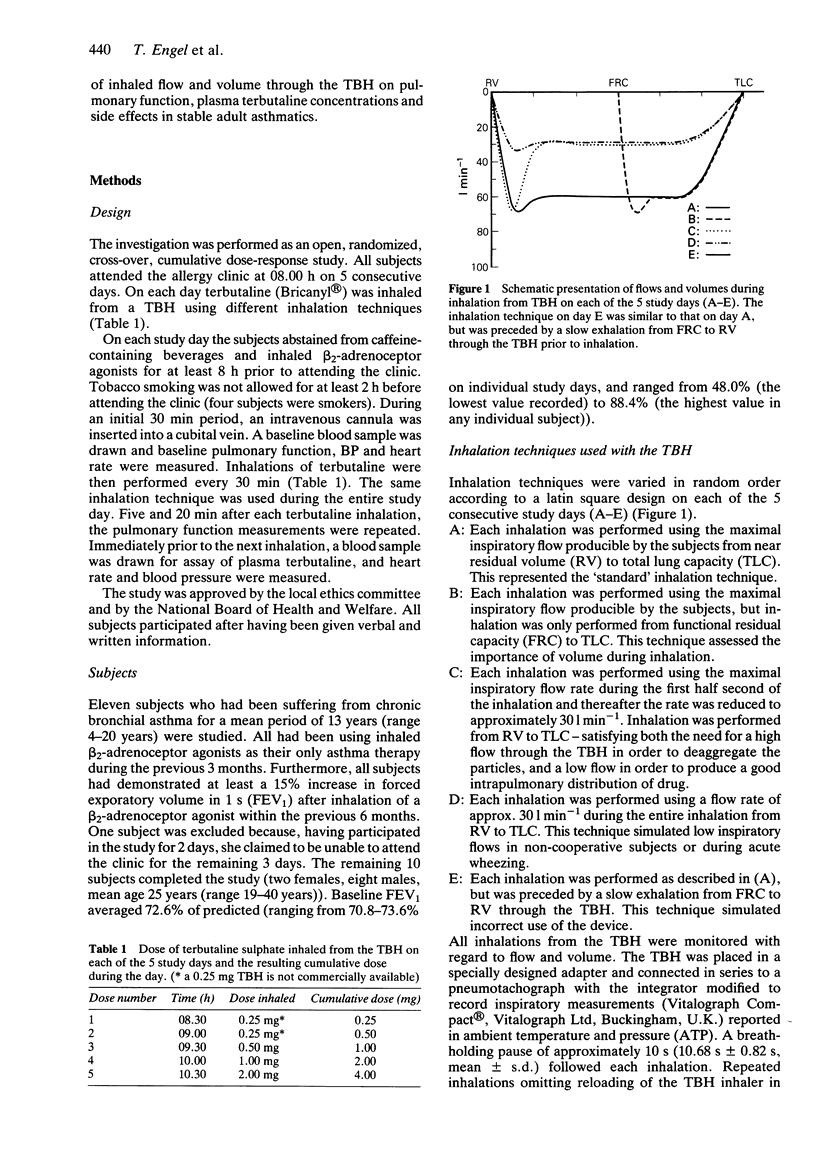
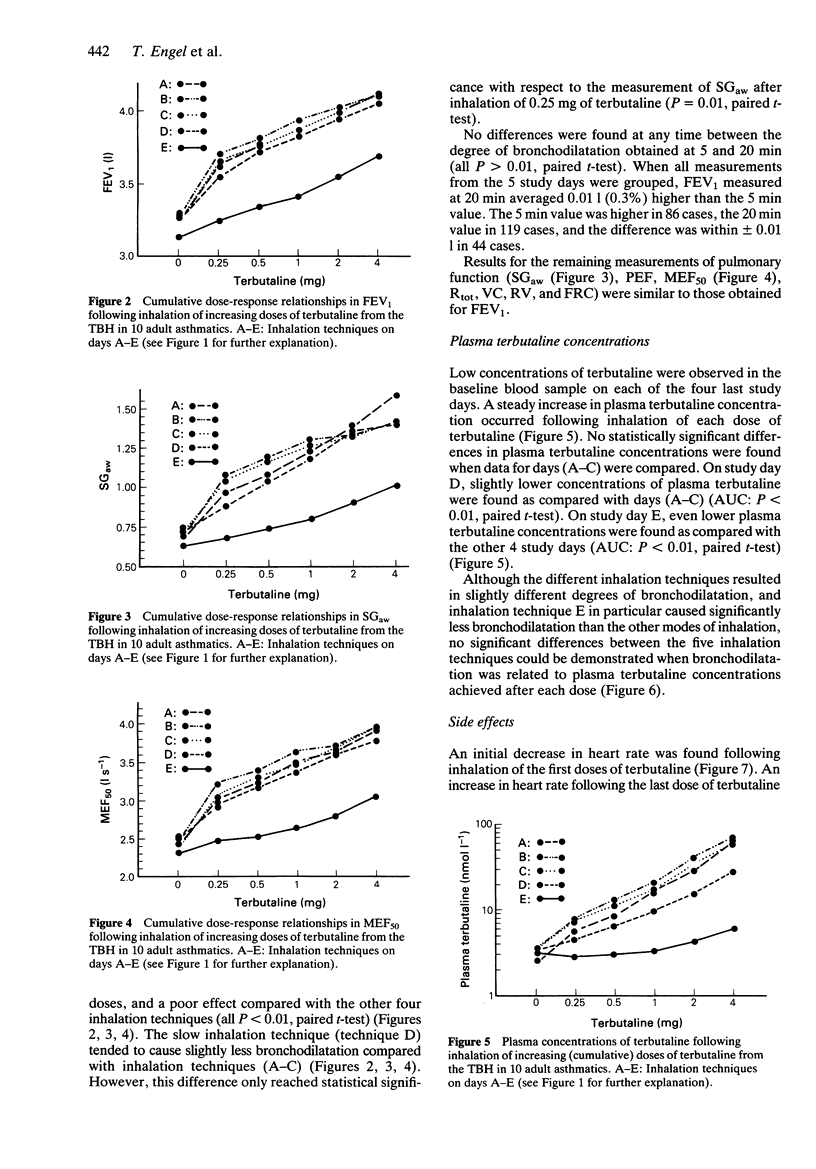
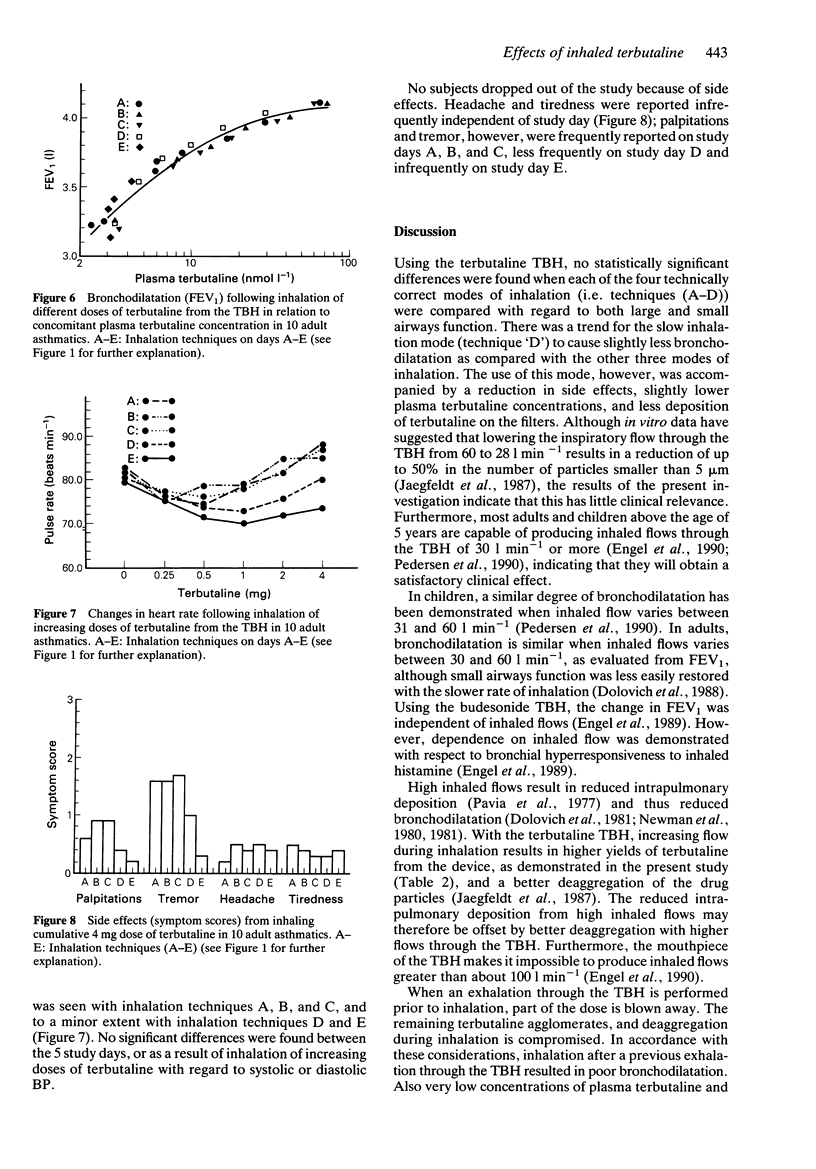
1. The efficacy of a metered dose inhaler (MDI) is highly dependent on the mode of inhalation. The relatively high built-in resistance in the Turbohaler (TBH), a new dry powder inhaler device for inhalation of terbutaline sulphate and budesonide, reduces the flow during inhalation. We compared five different modes of inhalation using the terbutaline TBH in 10 stable asthmatic subjects, who were tested on 5 consecutive days. 2. Measurement of 10 different parameters of pulmonary function indicated that the full bronchodilatory effect of an inhaled dose was already achieved at 5 min after the inhalation. Inspiratory flows through the TBH varying from 34 to 88 l min-1 resulted in comparable bronchodilation, and a previous exhalation to residual volume proved of no value. However, if, prior to inhalation, an exhalation through the device was performed, a substantially reduced effect was seen. 3. Reducing the inspiratory flow to approximately 34 l min-1 produced slightly reduced side effects and lower plasma terbutaline concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dolovich M., Ruffin R. E., Roberts R., Newhouse M. T. Optimal delivery of aerosols from metered dose inhalers. Chest. 1981 Dec;80(6 Suppl):911–915. [PubMed] [Google Scholar]
- Engel T., Heinig J. H., Madsen F., Nikander K. Peak inspiratory flow and inspiratory vital capacity of patients with asthma measured with and without a new dry-powder inhaler device (Turbuhaler). Eur Respir J. 1990 Oct;3(9):1037–1041. [PubMed] [Google Scholar]
- Engel T., Heinig J. H., Malling H. J., Scharling B., Nikander K., Madsen F. Clinical comparison of inhaled budesonide delivered either via pressurized metered dose inhaler or Turbuhaler. Allergy. 1989 Apr;44(3):220–225. doi: 10.1111/j.1398-9995.1989.tb02266.x. [DOI] [PubMed] [Google Scholar]
- Groth S., Dirksen H. Optimal inhalation procedure for the fenoterol powder inhaler. Eur J Respir Dis Suppl. 1983;130:17–24. [PubMed] [Google Scholar]
- Hansen O. R., Pedersen S. Optimal inhalation technique with terbutaline Turbuhaler. Eur Respir J. 1989 Jul;2(7):637–639. [PubMed] [Google Scholar]
- Johnsen C. R., Weeke E. R. Turbuhaler: a new device for dry powder terbutaline inhalation. Allergy. 1988 Jul;43(5):392–395. doi: 10.1111/j.1398-9995.1988.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Leferink J. G., Baillie T. A., Lindberg C. Quantitative analysis of terbutaline by gas chromatography-mass spectrometry. Eur J Respir Dis Suppl. 1984;134:25–32. [PubMed] [Google Scholar]
- Leferink J. G., Dankers J., Maes R. A. A time-saving method for the determination of the beta 2 sympathomimetics terbutaline, salbutamol and fenoterol. Preliminary results. J Chromatogr. 1982 Apr 16;229(1):217–221. doi: 10.1016/s0378-4347(00)86055-7. [DOI] [PubMed] [Google Scholar]
- Newman S. P., Morén F., Trofast E., Talaee N., Clarke S. W. Deposition and clinical efficacy of terbutaline sulphate from Turbuhaler, a new multi-dose powder inhaler. Eur Respir J. 1989 Mar;2(3):247–252. [PubMed] [Google Scholar]
- Newman S. P., Pavia D., Clarke S. W. Improving the bronchial deposition of pressurized aerosols. Chest. 1981 Dec;80(6 Suppl):909–911. [PubMed] [Google Scholar]
- Newman S. P., Pavia D., Clarke S. W. Simple instructions for using pressurized aerosol bronchodilators. J R Soc Med. 1980 Nov;73(11):776–779. doi: 10.1177/014107688007301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia D., Thomson M. L., Clarke S. W., Shannon H. S. Effect of lung function and mode of inhalation on penetration of aerosol into the human lung. Thorax. 1977 Apr;32(2):194–197. doi: 10.1136/thx.32.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Hansen O. R., Fuglsang G. Influence of inspiratory flow rate upon the effect of a Turbuhaler. Arch Dis Child. 1990 Mar;65(3):308–310. doi: 10.1136/adc.65.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Steffensen G. Fenoterol powder inhaler technique in children: influence of inspiratory flow rate and breath-holding. Eur J Respir Dis. 1986 Mar;68(3):207–214. [PubMed] [Google Scholar]
- Persson G., Gruvstad E., Ståhl E. A new multiple dose powder inhaler, (Turbuhaler), compared with a pressurized inhaler in a study of terbutaline in asthmatics. Eur Respir J. 1988 Aug;1(8):681–684. [PubMed] [Google Scholar]
- Richards R., Simpson S. F., Renwick A. G., Holgate S. T. Inhalation rate of sodium cromoglycate determines plasma pharmacokinetics and protection against AMP-induced bronchoconstriction in asthma. Eur Respir J. 1988 Dec;1(10):896–901. [PubMed] [Google Scholar]
- Sterling G. M., Batten J. C. Effect of aerosol propellants and surfactants on airway resistance. Thorax. 1969 Mar;24(2):228–231. doi: 10.1136/thx.24.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterlin K. Turbuhaler: a new powder inhaler for administration of drugs to the airways. Pharm Res. 1988 Aug;5(8):506–508. doi: 10.1023/a:1015969324799. [DOI] [PubMed] [Google Scholar]
- Yarbrough J., Mansfield L. E., Ting S. Metered dose inhaler induced bronchospasm in asthmatic patients. Ann Allergy. 1985 Jul;55(1):25–27. [PubMed] [Google Scholar]


