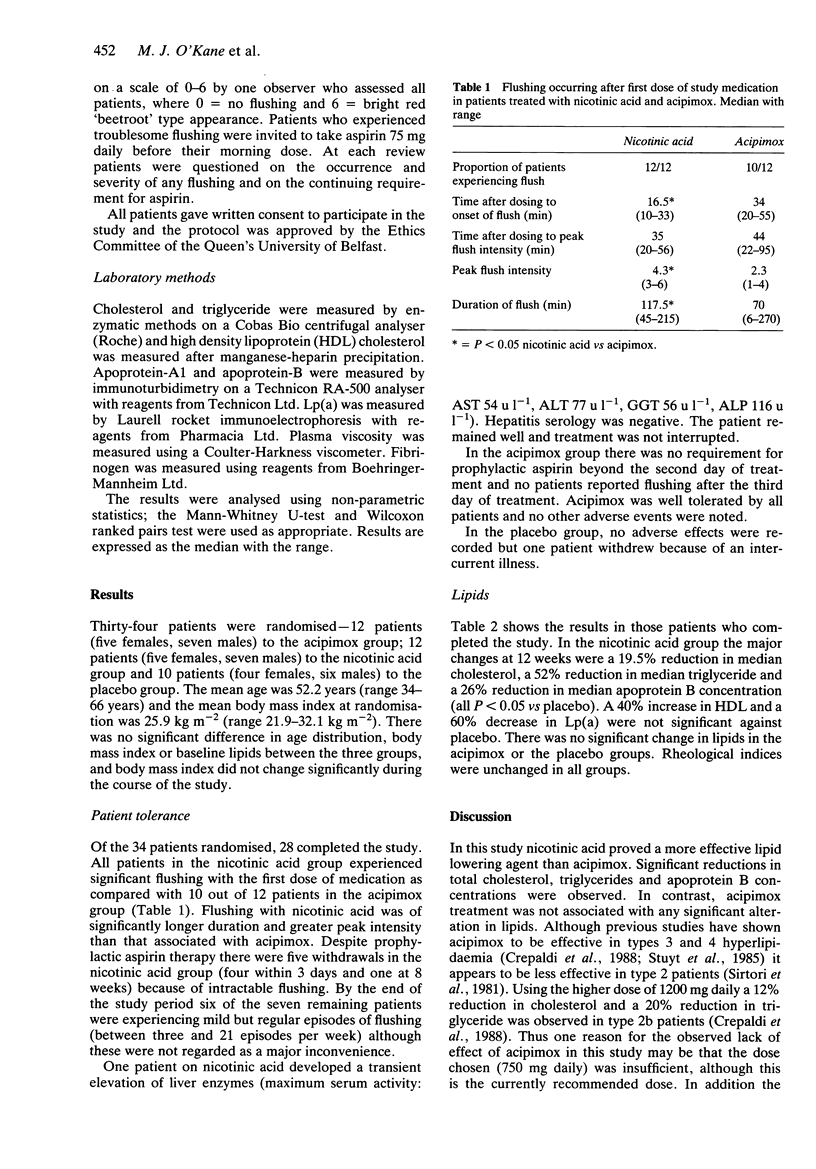
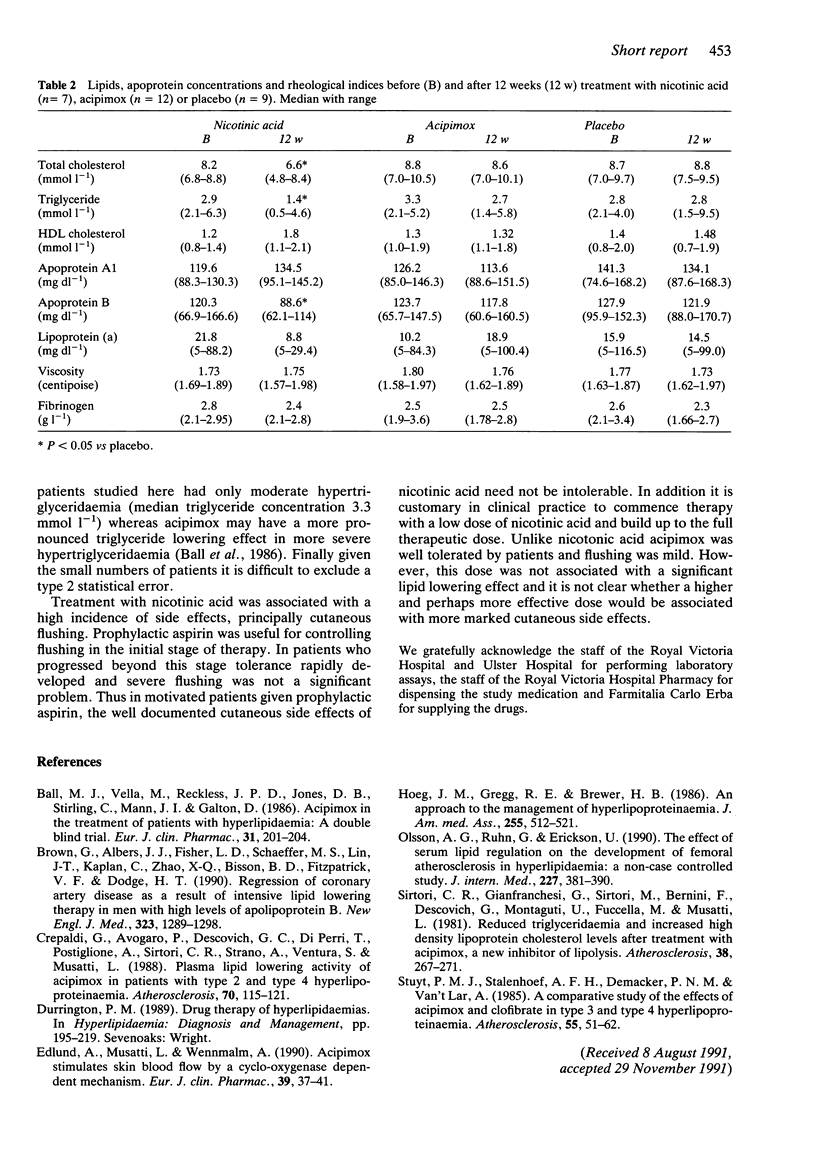
Abstract
The side effect profiles and lipid lowering efficacy of nicotinic acid (1 g three times daily) and its analogue acipimox (250 mg three times daily) in type 2b hyperlipidaemia were compared in a double-blind placebo controlled study. In the nicotinic acid group (n = 7) at 12 weeks there were significant reductions (P less than 0.05) with respect to placebo (n = 9) in total cholesterol (median and range) 6.6 mmol l-1 (4.8-8.4) vs 8.8 mmol l-1 (7.5-9.5), triglyceride 1.4 mmol l-1 (0.5-4.6) vs 2.8 mmol l-1 (1.5-9.5) and apoprotein B 88.6 mg dl-1 (62.1-114) vs 121.9 mg dl-1 (88.0-170.7). In contrast there was no significant alteration in lipids in the acipimox group (n = 12). Nicotinic acid was associated with a high incidence of side effects, principally cutaneous flushing, while acipimox was well tolerated by all patients.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball M. J., Vella M., Rechlass J. P., Jones D. B., Stirling C., Mann J. I., Galton D. Acipimox in the treatment of patients with hyperlipidaemia: a double blind trial. Eur J Clin Pharmacol. 1986;31(2):201–204. doi: 10.1007/BF00606659. [DOI] [PubMed] [Google Scholar]
- Brown G., Albers J. J., Fisher L. D., Schaefer S. M., Lin J. T., Kaplan C., Zhao X. Q., Bisson B. D., Fitzpatrick V. F., Dodge H. T. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990 Nov 8;323(19):1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- Crepaldi G., Avogaro P., Descovich G. C., Di Perri T., Postiglione A., Sirtori C. R., Strano A., Ventura S., Musatti L. Plasma lipid lowering activity of acipimox in patients with type II and type IV hyperlipoproteinemia. Results of a multicenter trial. Atherosclerosis. 1988 Mar;70(1-2):115–121. doi: 10.1016/0021-9150(88)90105-0. [DOI] [PubMed] [Google Scholar]
- Edlund A., Musatti L., Wennmalm A. Acipimox stimulates skin blood flow by a cyclo-oxygenase-dependent mechanism. Eur J Clin Pharmacol. 1990;39(1):37–41. doi: 10.1007/BF02657054. [DOI] [PubMed] [Google Scholar]
- Hoeg J. M., Gregg R. E., Brewer H. B., Jr An approach to the management of hyperlipoproteinemia. JAMA. 1986 Jan 24;255(4):512–521. [PubMed] [Google Scholar]
- Olsson A. G., Ruhn G., Erikson U. The effect of serum lipid regulation on the development of femoral atherosclerosis in hyperlipidaemia: a non-randomized controlled study. J Intern Med. 1990 Jun;227(6):381–390. doi: 10.1111/j.1365-2796.1990.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Sirtori C. R., Gianfranceschi G., Sirtori M., Bernini F., Descovich G., Montaguti U., Fuccella L. M., Musatti L. Reduced triglyceridemia and increased high density lipoprotein cholesterol levels after treatment with acipimox, a new inhibitor of lipolysis. Atherosclerosis. 1981 Feb-Mar;38(3-4):267–271. doi: 10.1016/0021-9150(81)90042-3. [DOI] [PubMed] [Google Scholar]
- Stuyt P. M., Stalenhoef A. F., Demacker P. N., Van 't Laar A. A comparative study of the effects of acipimox and clofibrate in type III and type IV hyperlipoproteinemia. Atherosclerosis. 1985 Apr;55(1):51–62. doi: 10.1016/0021-9150(85)90165-0. [DOI] [PubMed] [Google Scholar]


