Abstract
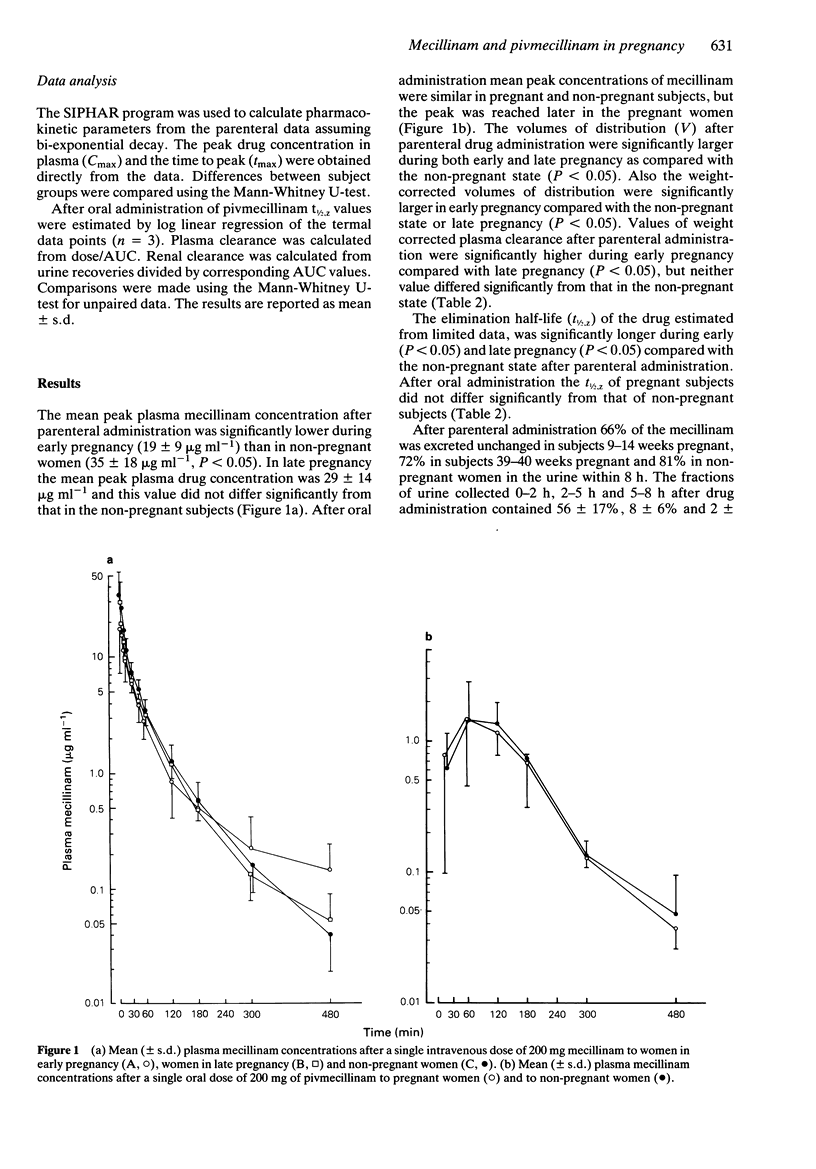
1. The pharmacokinetics of parenteral mecillinam (n = 27) and oral pivmecillinam (n = 12) were studied in pregnant (n = 27) and non-pregnant (n = 12) subjects. 2. In early pregnancy (9-14 weeks of gestation) the mean peak plasma drug concentration (Cmax = 19 +/- 9 micrograms ml-1) after an intravenous injection of 200 mg mecillinam was significantly lower (P less than 0.05) and the volume of distribution (V = 49 +/- 20.1) significantly larger (P less than 0.05) than in non-pregnant subjects (Cmax = 35 +/- 18 micrograms ml-1, V = 29 +/- 12.1). In late pregnancy (39-40 weeks of gestation) the plasma mean peak concentration (Cmax = (29 +/- 14 micrograms ml-1) after parenteral administration of 200 mg mecillinam was slightly lower and the volume of distribution (V = 65 +/- 29.1, V = 0.9 +/- 0.4 l kg-1) significantly larger than that in non-pregnant subjects (V = 0.4 +/- 0.3 l kg-1). Also after oral administration of 200 mg pivmecillinam, equimolar to 136.5 mg mecillinam, the mean peak plasma concentration in pregnant subjects (Cmax = 1.8 +/- 1.2 micrograms ml-1) was slightly lower than that in non-pregnant subjects (Cmax = 1.7 +/- 1.2 micrograms ml-1). 3. The mean half-life of elimination after parenteral administration of mecillinam was significantly longer during both early (t1/2,Z = 133 +/- 38 min, P less than 0.05) and late pregnancy (t1/2,Z = 107 +/- 41 min, P less than 0.05) as compared with the non-pregnant state (t1/2,Z = 75 +/- 21 min).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brumfitt W., Franklin I., Hamilton-Miller J., Anderson F. Comparison of pivmecillinam and cephradine in bacteriuria in pregnancy and in acute urinary tract infection. Scand J Infect Dis. 1979;11(4):275–279. doi: 10.3109/inf.1979.11.issue-4.04. [DOI] [PubMed] [Google Scholar]
- Cleeland R., Squires E. Enhanced activity of beta-lactam antibiotics with amdinocillin in vitro and and in vivo. Am J Med. 1983 Aug 29;75(2A):21–29. doi: 10.1016/0002-9343(83)90090-6. [DOI] [PubMed] [Google Scholar]
- Cox C. E. Parenteral amdinocillin for treatment of complicated urinary tract infections. Am J Med. 1983 Aug 29;75(2A):82–84. doi: 10.1016/0002-9343(83)90099-2. [DOI] [PubMed] [Google Scholar]
- Demos C. H., Green E. Review of clinical experience with amdinocillin monotherapy and comparative studies. Am J Med. 1983 Aug 29;75(2A):72–81. doi: 10.1016/0002-9343(83)90098-0. [DOI] [PubMed] [Google Scholar]
- Gambertoglio J. G., Barriere S. L., Lin E. T., Conte J. E., Jr Pharmacokinetics of mecillinam in health subjects. Antimicrob Agents Chemother. 1980 Dec;18(6):952–956. doi: 10.1128/aac.18.6.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godtfredsen W. O. An introduction to mecillinam. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):1–4. doi: 10.1093/jac/3.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- Heikkilä A., Erkkola R. Pharmacokinetics of piperacillin during pregnancy. J Antimicrob Chemother. 1991 Sep;28(3):419–423. doi: 10.1093/jac/28.3.419. [DOI] [PubMed] [Google Scholar]
- Kjer J. J., Ottesen B. Pharmacokinetics of pivmecillinam hydrochloride in pregnant and non-pregnant women. Acta Pharmacol Toxicol (Copenh) 1986 Nov;59(5):430–431. doi: 10.1111/j.1600-0773.1986.tb00195.x. [DOI] [PubMed] [Google Scholar]
- Lee T. L., Brooks M. A. Determination of amdinocillin in plasma and urine by high-performance liquid chromatography. J Chromatogr. 1982 Jan 8;227(1):137–148. doi: 10.1016/s0378-4347(00)80363-1. [DOI] [PubMed] [Google Scholar]
- Meyers B. R., Jacobson J., Masci J., Srulevitch E., Hirschman S. Z. Pharmacokinetics of amdinocillin in healthy adults. Antimicrob Agents Chemother. 1983 Jun;23(6):827–830. doi: 10.1128/aac.23.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Mecillinam--an amidino penicillin which acts synergistically with other beta-lactam compounds. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):43–52. doi: 10.1093/jac/3.suppl_b.43. [DOI] [PubMed] [Google Scholar]
- Reeves D. S. Antibacterial activity of mecillinam. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):5–11. doi: 10.1093/jac/3.suppl_b.5. [DOI] [PubMed] [Google Scholar]
- Reeves D. S., Wise R., Bywater M. J. A laboratory evaluation of a novel (beta-lactam antibiotic mecillinam. J Antimicrob Chemother. 1975 Sep;1(3):337–344. doi: 10.1093/jac/1.3.337. [DOI] [PubMed] [Google Scholar]
- Reynolds F., Knott C. Pharmacokinetics in pregnancy and placental drug transfer. Oxf Rev Reprod Biol. 1989;11:389–449. [PubMed] [Google Scholar]
- Sanderson P., Menday P. Pivmecillinam for bacteriuria in pregnancy. J Antimicrob Chemother. 1984 Apr;13(4):383–388. doi: 10.1093/jac/13.4.383. [DOI] [PubMed] [Google Scholar]
- Tybring L. Mecillinam (FL 1060), a 6beta-amidinopenicillanic acid derivative: in vitro evaluation. Antimicrob Agents Chemother. 1975 Sep;8(3):266–270. doi: 10.1128/aac.8.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]


