Abstract
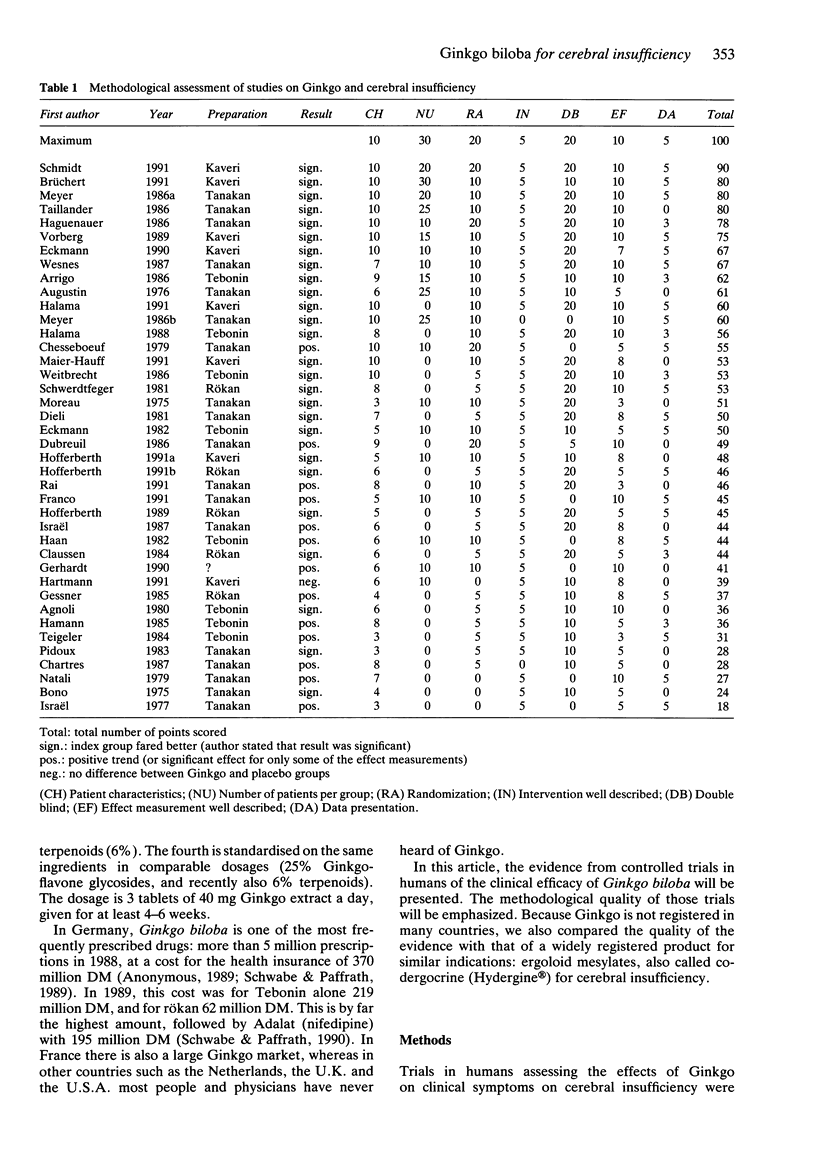
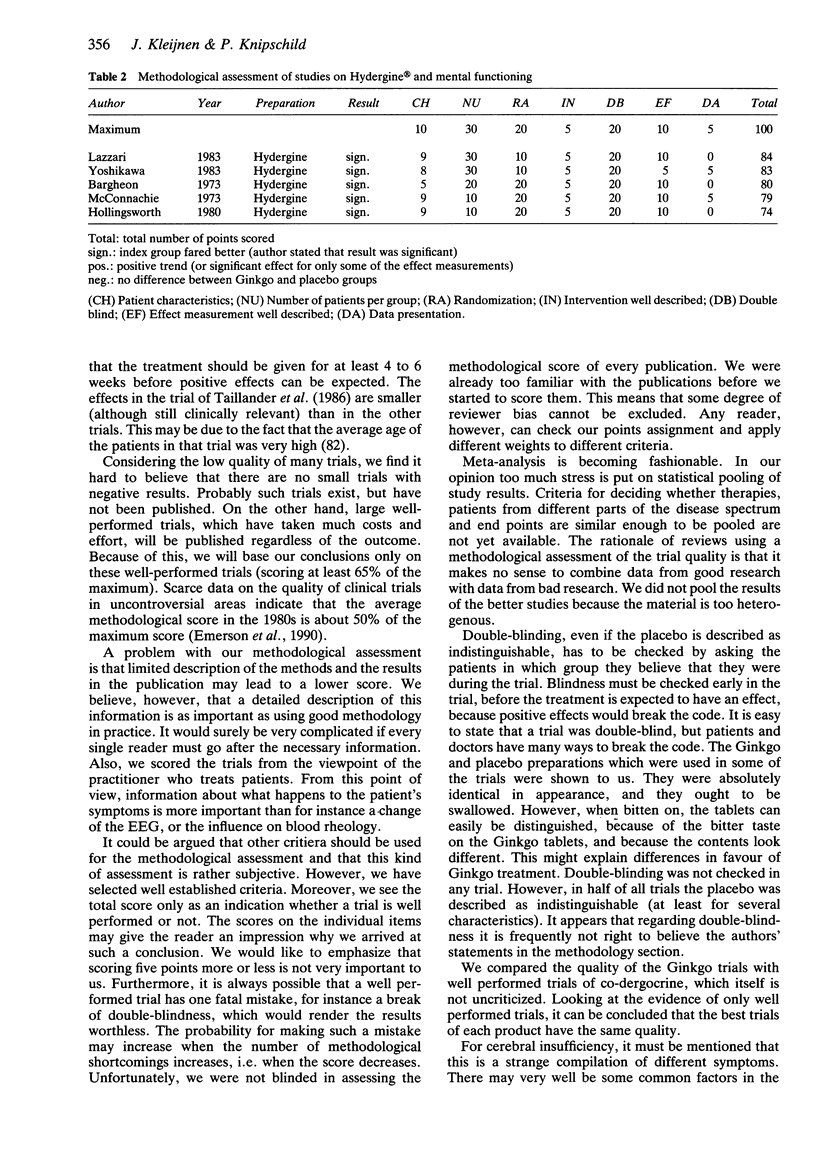
1. By means of a critical review we tried to establish whether there is evidence from controlled trials in humans on the efficacy of Ginkgo biloba extracts in cerebral insufficiency. 2. The methodological quality of 40 trials on Ginkgo and cerebral insufficiency was assessed using a list of predefined criteria of good methodology, and the outcome of the trials was interpreted in relation to their quality. A comparison of the quality was made with trials of co-dergocrine, which is registered for the same indication. 3. There were eight well performed trials out of a total of 40. Shortcomings were limited numbers of patients included, and incomplete description of randomization procedures, patient characteristics, effect measurement and data presentation. In no trial was double-blindness checked. Virtually all trials reported positive results, in most trials the dosage was 120 mg Ginkgo extract a day, given for at least 4-6 weeks. For the best trials, there were no marked differences in the quality of the evidence of the efficacy of Ginkgo in cerebral insufficiency compared with co-dergocrine. The results of the review may be complicated by a combination of publication bias and other biases, because there were no negative results reported in many trials of low methodological quality. 4. Positive results have been reported for Ginkgo biloba extracts in the treatment of cerebral insufficiency. The clinical evidence is similar to that of a registered product which is prescribed for the same indication. However, further studies should be conducted for a more detailed assessment of the efficacy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargheon J. Etude en double insu de l'hydergine chez le sujet âgé. Nouv Presse Med. 1973 Sep 8;2(31):2053–2055. [PubMed] [Google Scholar]
- Drieu K. Préparation et définition de l'extrait de Ginkgo biloba. Presse Med. 1986 Sep 25;15(31):1455–1457. [PubMed] [Google Scholar]
- Dubreuil C. Essai thérapeutique dans les surdités cochléaires aiguës. Etude comparative de l'extrait de Ginkgo biloba et de la nicergoline. Presse Med. 1986 Sep 25;15(31):1559–1561. [PubMed] [Google Scholar]
- Eckmann F. Hirnleistungsstörungen--Behandlung mit Ginkgo-biloba-Extrakt. Zeitpunkt des Wirkungseintrits in einer Doppelblindstudie mit 60 stationären Patienten. Fortschr Med. 1990 Oct 10;108(29):557–560. [PubMed] [Google Scholar]
- Eckmann F., Schlag H. Kontrollierte Doppelblind-Studie zum Wirksamkeitsnachweis von Tebonin forte bei Patienten mit zerebrovaskulärer Insuffizienz. Fortschr Med. 1982 Aug 26;100(31-32):1474–1478. [PubMed] [Google Scholar]
- Emerson J. D., Burdick E., Hoaglin D. C., Mosteller F., Chalmers T. C. An empirical study of the possible relation of treatment differences to quality scores in controlled randomized clinical trials. Control Clin Trials. 1990 Oct;11(5):339–352. doi: 10.1016/0197-2456(90)90175-2. [DOI] [PubMed] [Google Scholar]
- Gerhardt G., Rogalla K., Jaeger J. Medikamentöse Therapie von Hirnleistungsstörungen. Randomisierte Vergleichsstudie mit Dihydroergotoxin und Ginkgo-biloba-Extrakt. Fortschr Med. 1990 Jun 30;108(19):384–388. [PubMed] [Google Scholar]
- Gessner B., Voelp A., Klasser M. Study of the long-term action of a Ginkgo biloba extract on vigilance and mental performance as determined by means of quantitative pharmaco-EEG and psychometric measurements. Arzneimittelforschung. 1985;35(9):1459–1465. [PubMed] [Google Scholar]
- Haan J., Reckermann U., Welter F. L., Sabin G., Müller E. Ginkgo-biloba-Flavonglykoside. Therapiemöglichkeit der zerebralen Insuffizienz. Med Welt. 1982 Jul 9;33(27):1001–1005. [PubMed] [Google Scholar]
- Haguenauer J. P., Cantenot F., Koskas H., Pierart H. Traitement des troubles de l'équilibre par l'extrait de Ginkgo biloba. Etude multicentrique à double insu face au placebo. Presse Med. 1986 Sep 25;15(31):1569–1572. [PubMed] [Google Scholar]
- Halama P., Bartsch G., Meng G. Hirnleistungsstörungen vaskulärer Genese. Randomisierte Doppelblindstudie zur Wirksamkeit von Gingko-biloba-Extrakt. Fortschr Med. 1988 Jun 30;106(19):408–412. [PubMed] [Google Scholar]
- Hofferberth B. Einfluss von Ginkgo biloba-Extrakt auf neurophysiologische und psychometrische Messergebnisse bei Patienten mit hirnorganischem Psychosyndrom. Eine Doppelblindstudie gegen Plazebo. Arzneimittelforschung. 1989 Aug;39(8):918–922. [PubMed] [Google Scholar]
- Kleijnen J., Knipschild P., ter Riet G. Clinical trials of homoeopathy. BMJ. 1991 Feb 9;302(6772):316–323. doi: 10.1136/bmj.302.6772.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijnen J., Knipschild P., ter Riet G. Trials of homeopathy. BMJ. 1991 Apr 20;302(6782):960–960. doi: 10.1136/bmj.302.6782.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari R., Passeri M., Chierichetti S. M. Le mésylate de dihydroergotoxine dans le traitement de l'insuffisance cérébrale sénile. Résultat d'une étude clinique multicentrique en double aveugle contre placebo à long terme. Presse Med. 1983 Dec 29;12(48):3179–3185. [PubMed] [Google Scholar]
- McConnachie R. W. A clinical trial comparing 'Hydergine' with placebo in the treatment of cerebrovascular insufficiency in elderly patients. Curr Med Res Opin. 1973;1(8):463–468. doi: 10.1185/03007997309111708. [DOI] [PubMed] [Google Scholar]
- Meyer B. Etude multicentrique des acouphènes. Epidémiologie et thérapeutique. Ann Otolaryngol Chir Cervicofac. 1986;103(3):185–188. [PubMed] [Google Scholar]
- Meyer B. Etude multicentrique randomisée à double insu face au placebo du traitement des acouphénes par l'extrait de Ginkgo biloba. Presse Med. 1986 Sep 25;15(31):1562–1564. [PubMed] [Google Scholar]
- Moreau P. Un nouveau stimulant circulatoire cérébral. Nouv Presse Med. 1975 Oct 11;4(33):2401–2402. [PubMed] [Google Scholar]
- Rai G. S., Shovlin C., Wesnes K. A. A double-blind, placebo controlled study of Ginkgo biloba extract ('tanakan') in elderly outpatients with mild to moderate memory impairment. Curr Med Res Opin. 1991;12(6):350–355. doi: 10.1185/03007999109111504. [DOI] [PubMed] [Google Scholar]
- Schaffler K., Reeh P. W. Doppelblindstudie zur hypoxieprotektiven Wirkung eines standardisierten Ginkgo-Biloba-Präparates nach Mehrfachverabreichung an gesunden Probanden. Arzneimittelforschung. 1985;35(8):1283–1286. [PubMed] [Google Scholar]
- Subhan Z., Hindmarch I. The psychopharmacological effects of Ginkgo biloba extract in normal healthy volunteers. Int J Clin Pharmacol Res. 1984;4(2):89–93. [PubMed] [Google Scholar]
- Taillandier J., Ammar A., Rabourdin J. P., Ribeyre J. P., Pichon J., Niddam S., Pierart H. Traitement des troubles du vieillissement cérébral par l'extrait de Ginkgo biloba. Etude longitudinale multicentrique à double insu face au placebo. Presse Med. 1986 Sep 25;15(31):1583–1587. [PubMed] [Google Scholar]
- Weitbrecht W. U., Jansen W. Primär degenerative Demenz: Therapie mit Ginkgo-biloba-Extrakt. Plazebo-kontrollierte Doppelblind- und Vergleichsstudie. Fortschr Med. 1986 Mar 6;104(9):199–202. [PubMed] [Google Scholar]
- Yoshikawa M., Hirai S., Aizawa T., Kuroiwa Y., Goto F., Sofue I., Toyokura Y., Yamamura H., Iwasaki Y. A dose-response study with dihydroergotoxine mesylate in cerebrovascular disturbances. J Am Geriatr Soc. 1983 Jan;31(1):1–7. doi: 10.1111/j.1532-5415.1983.tb06280.x. [DOI] [PubMed] [Google Scholar]


